
Multiple faces of pulmonary large cell neuroendocrine carcinoma: update with a focus on practical approach to diagnosis
Introduction
Pulmonary large cell neuroendocrine carcinoma (LCNEC) represents a minority (~3%) of primary epithelial lung malignancies (1-3) that has been increasing in incidence over the last decade (4). Similar to small cell lung carcinoma (SCLC), the typical patient with LCNEC is an older man (median age of 65 years) with heavy smoking history, although most LCNEC are located in the lung periphery whereas most SCLC are central tumors (2,5). Patients with resectable early-stage disease have significant recurrence rate, generally reported as exceeding 50%; and many patients develop brain metastases (4,6). Patients presenting with distant metastases (stage IV) have very poor survival that is comparable to that of SCLC (2-5,7), although there are significant ranges of patient outcomes reported in different studies. Owing at least in part to its relatively low incidence, difficulty establishing pathologic diagnosis, and biologic heterogeneity, the optimal therapy for LCNEC is not well-established, and has been a topic of active clinical and scientific investigation over the past decade.
World Health Organization (WHO) definition
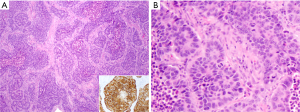
LCNEC has been grouped with other neuroendocrine (NE) tumors of the lung in the most recent 2015 WHO classification of thoracic tumors (8). The diagnostic criteria include cytologic features of non-small cell carcinoma (prominent nucleoli and/or abundant cytoplasm, and usually large cell size), NE architecture (organoid nesting, peripheral palisading, trabecular growth pattern, and/or rosette-like structures) with immunohistochemical (IHC) staining for one or more NE markers (synaptophysin, chromogranin, CD56, and more recently INSM1), and high mitotic activity (>10 per 2 mm2) with necrosis that is often geographic (Figure 1) (8). Although usually >1 NE marker is expressed in LCNEC and expression of at least one marker is typically diffuse (see below), in the presence of convincing NE morphology, any extent of NE marker reactivity can be accepted as supportive of LCNEC diagnosis. Although the presence of NE granules by electron microscopy is included in the diagnostic criteria, this approach has been virtually entirely supplanted by IHC in current practice. Any amount of morphologically recognizable adenocarcinoma (ADC), squamous cell carcinoma, giant cell carcinoma or spindle cell carcinoma in combination with LCNEC is sufficient for the diagnosis of combined LCNEC with the corresponding component. Combination with SCLC should be classified as combined SCLC and must contain at least 10% large cells.
Scenarios related to the diagnosis of LCNEC include cases with either NE morphology but lack of NE marker expression or vice versa. According to the WHO criteria, the tumors in the former category are classified as NSCLC or large cell carcinoma (LCC) with NE morphology (8). Given the expanding repertoire of NE markers in recent years with markers like INSM1 (9-11), this uncommon category may become even more rare, and be replaced with a more definitive diagnosis of LCNEC. Conversely, NE marker expression can occur in ~15% of NSCLC that otherwise lack NE morphology (2,11-18). As a group, such tumors are referred to as “NSCLC with NE differentiation” in the thoracic WHO classification. The significance of such expression remains unknown, with some studies showing that this bears no clinical implications (13,14,19). Such tumors should be diagnosed as ADC, squamous cell carcinoma or sarcomatoid/pleomorphic carcinoma (giant cell and/or spindle cell) based on the combination of defining morphologic features and corresponding non-NE immunomarkers, with a comment describing the positive NE markers. It is widely recommended that NE IHC should be avoided in cases with clear glandular, squamous, giant cell or spindle cell features in the absence of NE morphology (15).
Molecular update
Despite the remarkable advances in understanding of the molecular drivers of lung ADC, until recently, detailed molecular features of LCNEC have remained poorly characterized, largely owing to its relatively low prevalence and difficulty of initial diagnosis. In the last 5 years, a series of studies have been published, which utilized next-generation sequencing (NGS) to characterize the molecular landscape of LCNEC (20-24).
Briefly, the initial NGS study on LCNEC by Rekhtman et al. revealed that these tumors are molecularly heterogeneous and can be classified into two major subsets—small cell-like LCNEC (SC-LCNEC) and non-small cell-like LCNEC (NSC-LCNEC)—depending upon the major molecular alterations (Figure 2) (20). SC-LCNEC subset was characterized primarily by RB1 (retinoblastoma) and TP53 (tumor protein p53) inactivation, whereas NSC-NSCLC subset was associated with KRAS, STK11 (serine/threonine kinase 11) or KEAP1 (kelch like ECH associated protein 1) mutations alone or concurrently with TP53 mutations. Additional less common molecular alterations seen almost exclusively in the SC-LCNEC included MYCL1 amplification and PTEN mutations, and those seen exclusively in the NSC-LCNEC involved BRAF, MAP2K1, ERBB2, and CDKN2A genes. Furthermore, a small subset of carcinoid-like LCNECs was identified, which was characterized by MEN1 alterations and lack of RB1/TP53 alterations (see section on highly-proliferative carcinoids below) (20).
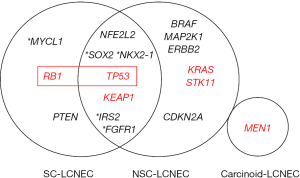
Subsequently, a pivotal study by George et al., which combined whole-exome/genome sequencing and transcriptomic analysis, identified similar SC- and NSC-genomic subsets in LCNEC, which were designated “type II LCNEC” and “type I LCNEC”, respectively. Interestingly, at the transcriptional level, LCNEC with SC-type genomic alterations (type II) were largely distinct from conventional SCLC, whereas LCNEC with NSC-type genomic alterations (type I) exhibited transcriptional similarities to SCLC (23). These findings support that despite genomic overlap with SCLC and NSCLC, the respective subsets of LCNEC do represent distinct entities from their conventional counterparts, and that phenotypes of LCNEC are a result of a complex interplay of genomic and transcriptional programs. Overall, these molecular data provide evidence for biological heterogeneity of LCNEC, and likely reflect distinct cells of origin and pathogenesis of LCNEC subsets merging in the final common phenotypic appearance of LCNEC.
Regarding the question of whether genomic subsets correspond to specific histopathologic features of LCNEC, the initial study suggested that indeed tumors with SC-like genomic profiles tended to have higher Ki-67 rates and a spectrum of morphologic features closer to SCLC (including greater cell crowding and overall smaller cell size) than LCNEC with NSC-like alterations (20). Conversely, low-level expression of exocrine marker Napsin A was present exclusively in a minority of LCNECs with NSC-like genomic profiles. However, both proliferation rate and morphologic features had substantial overlap between these two molecular subsets. Thus, morphology alone does not fully predict the genomic features. However, the status of RB1 and TP53—the major defining alterations of the SC- versus NSC-LCNEC subsets—can be assessed by IHC, and can thus serve as a surrogate for molecular testing. In recent studies, LCENC subsets defined by RB1 and TP53 alteration using molecular and IHC methods were found to show prognostic and therapeutic differences, as discussed further below (25,26). However, routine use of these markers in clinical practice awaits robust confirmation of clinical utility, including analysis incorporating a combination of genomic profiles, morphologic features, and proliferation rates with clinical outcomes.
Clinical update
Historically, data on systemic therapeutic approaches to stage IV LCNEC has been conflicting, with some studies suggesting benefits of etoposide/platinum regimens used in the treatment of SCLC, and others showing benefits of NSCLC-type therapy (6,7,27-31). More recent data have suggested improved treatment response and survival benefits in patients treated according to the molecular subtype (25,26,32). Most recently, in a study by Zhuo et al. (26), despite having a shorter overall survival, more patients with SC-LCNEC responded either completely or partially to the conventional SCLC chemotherapy than those with NSC-LCNEC (47% vs. 26%, respectively). However, even in SC-LCNEC, the response rate to platinum/etoposide was lower than historically reported for conventional SCLC (~70%). These findings corroborate prior studies, including that by Naidoo et al., which also found that LCNEC response to chemotherapy was worse than that for SCLC (6). Unfortunately, the major conclusion from the above studies was that patients with advanced LCNEC have extremely poor prognosis irrespective of the type of chemotherapy or molecular subset. In recent years, immunotherapy has become the mainstay of therapy for NSCLC (33) and was also recently approved for SCLC (34). Efficacy of immunotherapeutic agents in LCNEC awaits assessment in clinical studies, but robust responses to checkpoint inhibitors have been reported in small series (35,36).
Early-stage LCNEC is managed similarly to NSCLC in that localized stage I and II tumors are resected. Overall, the prevalence of early-stage LCNEC significantly exceeds that of SCLC, which is only exceptionally diagnosed at an early-stage. In such instances, unlike LCNEC, resection of SCLC is only recommended for stage I/II N0 disease (37). A recent large retrospective study suggested a survival benefits of surgery in patients with LCNEC up to and including clinical stage IIIA (38). Thus, accurate initial diagnosis of LCNEC vs. SCLC may become increasingly important for the decision on surgical management in patients with locally-advanced disease.
Differential diagnostic considerations for LCNEC: the multiple faces
Given the wide morphologic spectrum of LCNEC, a number of entities enter in the differential diagnosis with LCNEC. The main entities include SCLC, atypical carcinoid, basaloid squamous cell carcinoma (BSCC), and solid and/or cribriform ADC or LCC, the latter of which is an extremely rare diagnostic category for fully resected NSCLC lacking morphologic and IHC evidence of glandular or squamous differentiation (39,40). Another important differential diagnostic consideration includes a recently described entity of SMARCA4-deficient undifferentiated thoracic tumors (SD-UTT) with round cell/rhabdoid features. Various morphologic and IHC features of LCNEC overlap with these entities, and in practice, the diagnosis of LCNEC continues to present a challenge, even among expert thoracic pathologists (41-43). Although the most widely recognized and most commonly discussed diagnostic challenge is between LCNEC and SCLC, other aforementioned differentials are also commonly encountered in practice.
This review is aimed at addressing the practical approach to diagnostic challenges with LCNEC using illustrative examples of the main differential diagnoses. Clinical, pathologic and molecular aspects relevant to each case will be discussed.
Diagnostic challenges
LCNEC versus small cell lung carcinoma (SCLC)
Perhaps the most challenging and most widely recognized differential diagnosis of LCNEC is that of SCLC, which is evidenced by variable interobserver reproducibility for distinguishing these entities (41,43,44). The distinction relies upon a combination of morphometric features including cell shape and size, amount of cytoplasm, nuclear-to-cytoplasmic (N:C) ratio, chromatin quality and nucleolar prominence. LCNEC is characterized by larger cell size, moderate-to-large amount of cytoplasm, polygonal cell shape, lower N:C ratio, coarsely granular or vesicular chromatin, and usually prominent nucleoli (Figure 3). Conversely, the cells in SCLC are smaller and round to fusiform, with scant cytoplasm, very high N:C ratio, generally smooth nuclear contours, finely granular chromatin, and inconspicuous or absent nucleoli (Figure 4). Although objective definition of “prominent nucleoli” and “moderate to abundant cytoplasm” is lacking, we find helpful in our practice to regard nucleoli as prominent if they are visible from medium magnification with a 10× objective, and to consider cytoplasm as abundant when inter-cellular borders are readily visible.
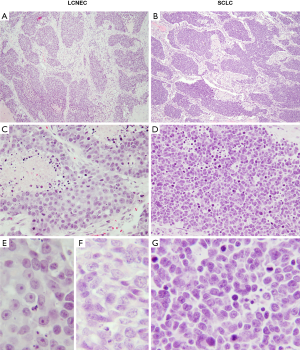
The accuracy of the diagnosis can be further complicated by significant crush artifact seen frequently on endobronchial and transbronchial biopsies (Figure 3), which are commonly used for sampling of central lung tumors. The cells can appear small and the artifactual overlap between the cells can mimic nuclear molding (Figure 3A). These features, in the presence of NE marker immunoreactivity and a high (50–100%) Ki-67 proliferative index (Figure 3B,C,D), can make the distinction between LCNEC and SCLC nearly impossible. Careful examination of all parts of the biopsy on intermediate or high power to look for areas with preserved cytomorphology is critical to make the correct diagnosis. If a crushed biopsy lacks areas with well-preserved morphology, the diagnosis of high-grade NE carcinoma with a comment explaining the possibility of both entities is most appropriate.
Typically, small cell carcinomas are composed of infiltrating sheets of small blue cells with broad areas of necrosis and prominent apoptotic activity. However, nested architecture may also be seen (Figure 4A), as can trabecular growth, peripheral palisading and rosette-like structures (45). These features are usually best appreciated on resections—a rare specimen type for SCLC given that these tumors usually present at advanced, unresectable stage. The presence of at least focal nested architecture has been reported in 94% of resected SCLC (46), yet given the rarity of such specimens pathologists may not be familiar with the potential of SCLC to exhibit nested/organoid architecture. This can lead to mis-interpretation of SCLC with nested pattern as LCNEC. Furthermore, although typically described as less than the diameter of three lymphocytes, SCLC cells can display a range of sizes measuring up to the size of LCNEC (45,47-49). Fusiform cell shape and venous incrustation with basophilic nuclear material (Azzopardi phenomenon) are more characteristic of SCLC, but the diagnosis ultimately rests upon the three main cytologic features: amount of cytoplasm, nucleolar prominence, and chromatin pattern (Figures 3E,F,4B,C,D,E,F,G). If any amount of tumor has classical small cell carcinoma cytologic features in the background of LCNEC, the diagnosis of combined SCLC with LCNEC is recommended by the WHO (8,50). It is important to acknowledge that there is a morphologic spectrum in both SCLC and LCNEC, and some cases are difficult to distinguish, though it is suggested that this should apply to approximately 5% of well-preserved specimens (51).
Immunohistochemistry has only a limited role in distinguishing LCNEC and SCLC. One potentially helpful but low-sensitivity feature is focal and weak Napsin A immunoreactivity, which can be seen in up to 15% of LCNEC (52,53), whereas SCLC is consistently negative for this marker. Thus, when present, weak Napsin A labeling in a high-grade NE carcinoma may favor the diagnosis of LCNEC. Additionally, diffuse membranous cytokeratin immunoreactivity has been suggested to favor the diagnosis of LCNEC since SCLC classically has weak dot-like keratin reactivity. However, the dot-like and/or weak staining pattern can also be seen in LCNEC (44,54). Thus, this feature is not widely used in practice.
Genomic overlap between LCNEC and SCLC (Figure 2) (20-24) makes the distinction between these entities possible by molecular studies only in cases with NSCLC-like mutational profiles (i.e., NSC-LCNEC subset described above). LCNECs with a SC-like molecular profile (SC-LCNEC) are genomically indistinguishable from SCLC. Overall, potential utility of genomic studies for the distinction between SCLC and LCNEC requires further analysis.
In summary, there is currently no entirely sensitive and specific IHC or molecular marker to separate LCNEC and SCLC, and this remains a distinction that relies entirely on morphologic criteria. Development of objective biomarkers specific for SCLC vs. LCNEC would allow greater diagnostic reproducibility. Additionally, development of predictive biomarkers of response to specific systemic therapies—potentially irrespective of LCNEC vs. SCLC diagnosis—would also allow objective criteria for guiding treatment decisions. For example, SLFN11 has recently emerged as a promising marker of sensitivity to cytotoxic agents in SCLC (55-57). Its utility in LCNEC and validation of the utility in clinical practice requires further studies.
LCNEC versus adenocarcinoma (ADC) or large cell carcinoma (LCC)
Since the diagnosis of LCNEC by definition requires the presence of non-small cell carcinoma cytologic features, differentiating solid and/or cribriform ADC or LCC from LCNEC relies on the presence of classical NE architecture and confirmation of this morphologic impression by expression of NE markers. However, architectural features can occasionally be equivocal for LCNEC versus solid/cribriform ADC or LCC with nested organoid-like pattern. Furthermore, as mentioned above, ~15% of ADC/LCC have NE marker immunoreactivity in the absence of NE morphology (13,14,17-19). Thus, distinction of these entities can cause a diagnostic dilemma, particularly in small biopsies or cytology samples.
A helpful consideration for this differential diagnosis is the extent and number of positive NE markers, which is generally significantly different between LCNEC and ADC/LCC with NE marker expression. Most LCNEC show expression of two to three standard NE markers (synaptophysin, chromogranin, CD56), and expression of at least one NE marker is typically diffuse (3,20,53,58). However, ~20% of LCNEC are reported to label for only one NE marker, with some cases showing only focal immunoreactivity for a single marker (53,58). Conversely, NE marker expression in ADC/LCC is usually focal and typically limited to only one marker (11-14,16,19,53,58,59); however, expression of two NE markers has been reported in 1–4% of ADC (16,19). Thus, while diffuse NE marker immunoreactivity and/or expression of ≥2 markers is substantially more common in LCNEC, lower levels of NE marker expression overlap with ADC/LCC. In such cases, the diagnosis hinges on the impression of NE morphology.
A helpful morphologic feature for distinguishing LCNEC with prominent rosette-like structures from cribriform ADC is the shape of the luminal spaces (Figure 5). While the rosette-like structures of LCNEC are characterized by punched out lumens (resembling breast DCIS) (Figure 5A), the cribriform spaces in ADC are often more collapsed (resembling usual ductal hyperplasia) (Figure 5B). Overall, ADCs tend to have more voluminous eosinophilic cytoplasm, whereas the cytoplasm in LCNEC tends to be more scant and commonly has amphophilic quality.
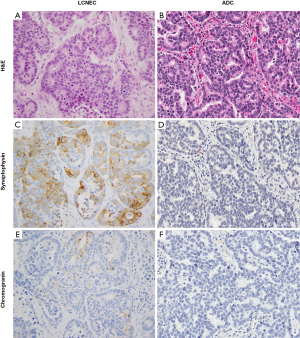
Similar to the differential diagnosis with SCLC, there is no single defining IHC marker for distinguishing LCNEC from ADC/LCC with NE marker expression. However, several IHC features can provide supporting evidence in favor of one versus the other diagnosis, in addition to the extent and number of expressed NE markers. Namely, as mentioned above, while focal and weak Napsin A immunoreactivity can be seen in up to 15% of LCNEC (52,53), diffuse and strong expression of this marker is unusual for this diagnosis and favors the diagnosis of ADC. Similarly, relative amounts of TTF-1 to Napsin A expression can be used as a supportive feature: strong and diffuse TTF-1 immunoreactivity in conjunction with negative or very focal/weak staining with Napsin A is a soft feature for LCNEC since most ADCs with strong/diffuse expression of TTF-1 also have strong expression of Napsin A, although there are exceptions (52,53). Another relatively helpful but neither fully sensitive nor specific feature is the Ki-67 proliferative index. While very high Ki-67 (80–100%) is common in LCNEC, this level of proliferative activity is unusual for ADC/LCC (8,53,60). It is important to note that while these additional IHC features can serve as supporting observations, they are not diagnostic in isolation. Finally, genomic characteristics are insufficient to distinguish LCNEC (specifically NSC-like subset) from ADC/LCC.
It has been suggested that ADC/LCC with NE marker expression may exist in a biologic continuum with NSC-LCNEC, ranging from cases having only weak NE marker expression in the absence of NE morphology to those exhibiting a full set of morphologic and marker expression characteristics of LCNEC in a genomic background of ADC. Diagnostic challenges in a minority of cases may thus reflect this biological continuum (52).
The distinction of LCNEC from solid/cribriform ADC or LCC with NE marker expression is particularly challenging in small biopsies. It is well recognized that the approach to this differential diagnosis is an integrative one, requiring consideration of all the aforementioned factors. The diagnosis of LCNEC on biopsies has been considered controversial given the difficulty in appreciating NE morphology in high-grade carcinoma in small or fragmented tissue. However, in recent years it has become standard to obtain larger amounts of tissue for thoracic biopsies given increased tissue demands for molecular studies. This allows greater ability to discern NE morphology, and suggest the diagnosis of LCNEC. In a recent study by Baine et al., NE morphologic features were evident in 85% of 1 mm tissue cores from LCNEC (53). In that study, the proposed integrative semiquantitative scoring criteria combining NE morphology (peripheral palisading, rosette-like structures, organoid nesting, and extensive necrosis), NE marker expression and Ki-67 proliferative index was highly sensitive and specific for distinguishing LCNEC from ADC/LCC. Previously, Derks et al. had suggested that the presence of ≥2 positive NE markers in NSCLC is by itself sufficient to make the diagnosis of LCNEC in a biopsy specimen lacking glandular or squamous differentiation even in the absence of NE morphology (58). However, this method may miss a subset of LCNEC with focal and/or single NE marker immunoreactivity, which can occur in up to 20% of cases (53). Furthermore, expression of ≥2 NE markers, particularly at a low level, can be encountered in a minority of ADC/LCC cases (16,19). Therefore, in our practice we currently limit the use of NE markers in well-preserved specimens to tumors with at least some suggestion of NE morphology. We note that even in the absence of definitive evidence of palisading or rosettes, LCNEC may be suspected in biopsies of high-grade malignant tumors with even subtle nested architecture, cytoplasmic amphophilia and/or stippled chromatin.
In summary, differentiation of LCNEC from ADC/LCC requires a combination of NE morphology and marker expression. In cases where the morphology is equivocal, extent of NE marker reactivity, very high Ki-67 and negative or low Napsin A expression relative to high TTF-1 reactivity can serve as supporting (but not in isolation diagnostic) evidence for LCNEC over ADC/LCC.
LCNEC versus basaloid squamous cell carcinoma (BSCC)
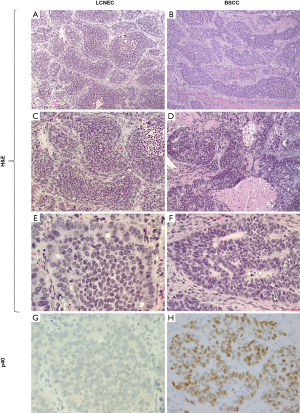
Nested growth pattern with peripheral palisading, high N:C ratios imparting a “blue look” from low power, and large zones of necrosis are the morphologic features shared between LCNEC and BSCC (Figure 6A,B,C,D). Furthermore, some BSCC show prominent rosette-like structures similar to those seen in LCNEC (Figure 6D,E,F). Presence of focal keratinization is diagnostic of BSCC even without confirmatory IHC, but keratinization may be entirely absent in a subset of BSCC. Other distinctive morphologic features are the presence of hyaline stroma and basement membrane-like material in BSCC (Figure 6D,F). Unlike the lack of distinguishing IHC markers for the differential diagnosis of LCNEC with SCLC and the potential overlap between LCNEC and ADC/LCC with NE differentiation, there are reliable and objective markers for the distinction between BSCC and LCNEC. Namely, BSCC consistently express squamous marker p40 (Figure 6G,H), whereas diffuse expression of this marker is not expected in LCNEC. However, focal p40 staining in scattered cells does occur in some LCNEC (20). Although p63 and CK5/6 are highly sensitive squamous markers, they are much less specific than p40, and CK5/6 is also less sensitive; thus, they provide limited to no additional information if p40 is available (61,62). Notably, a potential pitfall in this differential diagnosis is NE marker expression (especially CD56) in BSCC, which has been reported in a subset of these tumors (63-65). However, the diagnosis in this setting can be readily clarified by diffuse expression of p40 and attention to the aforementioned distinctive morphologic characteristics.
LCNEC versus atypical carcinoid
Carcinoid tumors have sharply distinct epidemiology and molecular pathogenesis from LCNEC (and SCLC). Compared to LCNEC/SCLC, they lack strong association with smoking, tend to arise in younger patients, and have significantly more indolent behavior even in stage IV setting. Molecularly, these tumors have low tumor mutation burden and commonly harbor MEN1 mutations, but lack RB1 or TP53 alterations (66). However, a recent study suggested that a small subset of atypical carcinoids has gene expression and methylation profiles resembling LCNEC (so-called “supra-carcinoids”) (67). However, pathological and clinical characteristics of such tumors remain to be defined.
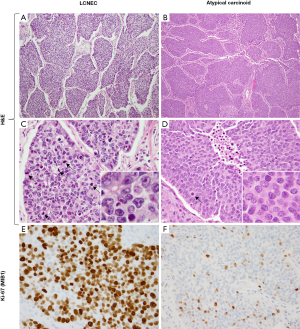
By the 2015 WHO criteria, the key distinguishing feature between LCNEC and atypical carcinoids is the mitotic rate of >10 per 2 mm2. However, this distinction also implies characteristic cytomorphologic criteria (Figures 7,8). The prototypical LCNEC shows nuclear pleomorphism, large cell size, nuclear membrane irregularities, coarse or vesicular chromatin, and prominent nucleoli (Figure 7C). In contrast, typical cytologic features of carcinoid tumors include cellular uniformity and monotony, relatively small cell size, smooth nuclear borders, salt-and-pepper chromatin and lack of prominent nucleoli (Figure 7D). It is important to note that similar to other endocrine tumors, marked cytologic atypia may occur in carcinoid tumors, typical and atypical alike, but it is usually focal/random and seen in the background of the more conventional monotonous cytomorphology. While small nucleoli can be present in atypical carcinoids, they should not be a conspicuous feature. Conversely, although some LCNECs may have nuclear monotony, they typically have more marked nuclear membrane irregularities/convolution and more prominent nucleoli.
Necrosis is another distinctive feature, in that it is characteristically punctate/comedo-like in atypical carcinoids (Figure 7D) versus geographic in LCNEC. However, necrosis in some LCNECs may also be limited to punctate areas. Thus, the diagnosis of LCNEC should not be excluded in the absence of broad areas of necrosis.
In contrast, NE architecture is a shared feature between LCNEC and carcinoid tumors. In fact, the concept of NE architecture in LCNEC is adopted from the type of morphologic patterns that can be encountered in carcinoids and well-differentiated neuroendocrine tumors (WD-NETs) of other organs. Both tumors are typically nested (Figures 7A,B,8B,C) and have prominent rosette-like structures (Figures 5A,8D), trabecular growth pattern, and peripheral palisading.
In most cases of resected primary tumors, the distinction between LCNEC and atypical carcinoids is evident based on the aforementioned cytologic features and the nature of necrosis. Generally, morphologic impression of LCNEC is accompanied by mitotic counts significantly exceeding the threshold of 10 mitoses per 2 mm2, whereas the converse is true for atypical carcinoids. Although Ki-67 is not formally included in the 2015 WHO classification of lung NE tumors, it is commonly used in the interpretation of biopsies and cytology specimens (53,69-75), where mitotic counts are more difficult to assess than Ki-67 rate.
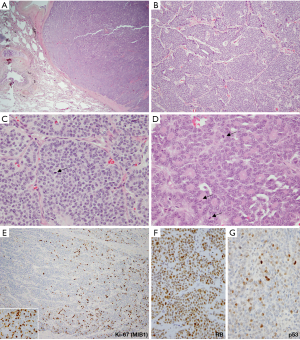
Although there is typically a sharp separation of both mitotic and Ki-67 rates in LCNEC versus atypical carcinoids, in recent literature the issue of proliferative overlap among these tumors has started to emerge (76-79). Thus, while most LCNEC have 60–80 mitoses per 2 mm2, tumors with intermediate mitotic rates do occur, including those approaching 20 per 2 mm2. For Ki-67, LCNEC shows rates that are typically >50% (Figure 7E), but some cases have intermediate rates in the 20–50% range. Conversely, while the majority of atypical carcinoids have mitotic counts below 10 per 2 mm2 and Ki-67 of <20% (usually <10%; Figure 7F), recently data is emerging on the expanded proliferative spectrum in lung carcinoids, particularly in the metastatic setting. More specifically, recent studies have described tumors with morphologic features of carcinoid tumors, but mitotic counts exceeding the atypical carcinoid threshold of 10 per 2 mm2 and/or surpassing the typical Ki-67 rates of under 20%. However, in most such cases mitotic and Ki-67 rates did not exceed 20 per 2 mm2 and 50%, respectively. Furthermore, unlike LCNEC (and SCLC), which typically show uniformly high mitotic counts and Ki-67 (Figure 7E), these tumors characteristically have marked intratumor proliferative heterogeneity, featuring proliferative hotspots and other areas of low proliferation rates (Figure 8E). While such tumors are rare among primary carcinoids, a recent study in stage IV lung carcinoids found that proliferative progression with escalation of proliferation rates above the standard thresholds is seen in ~25% of metastatic samples (68).
Although the current WHO classification regards tumors with morphologic features of atypical carcinoids but mitotic counts >10 per 2 mm2 as LCNEC, recent studies suggest that their clinicopathologic and molecular characteristics are akin to carcinoids rather than NE carcinomas (68,76-79). These concepts are analogous to the recent category of WD-NET, grade 3 in the enteropancreatic system, where such tumors were also previously classified as NE carcinomas. The approach to these tumors awaits further studies and the updated thoracic WHO classification.
Presently, for diagnostic purposes, it is important to be aware of these recent data on the expanded proliferation spectrum in carcinoid tumors and potential mitotic and Ki-67 overlap with LCNEC, especially in the metastatic setting. This particularly applies to tumors with intermediate proliferation rates (10–20 mitoses per 2 mm2 and/or with Ki-67 of 20–50%). Interpretation of biopsies falling into this “gray-zone” can be challenging. Similar to the pancreatic WD-NET, grade 3, the assessment of RB and p53 by IHC (Figure 8F,G) has been suggested to be of potential utility (80,81), since these genes are frequently mutated in LCNEC but they consistently show wild-type pattern in carcinoid tumors (68); however, this requires further validation. Conversely, while IHC for DAXX (death-domain associated protein) and ATRX (alpha thalassemia mental retardation syndrome X-linked) has been used to distinguish pancreatic WD-NET and NEC since ~40% of pancreatic WD-NETs harbor inactivating mutations/loss of expression of these markers (81,82), alterations in these genes are only rarely seen in pulmonary carcinoid tumors (66,67). The utility of comprehensive genomic analysis for distinguishing highly-proliferative carcinoids and LCNEC in biopsies (and rarely resected tumors) falling in the proliferative gray-zone will be of interest to explore in future studies.
LCNEC versus SMARCA4-deficient undifferentiated thoracic tumor (SD-UTT)
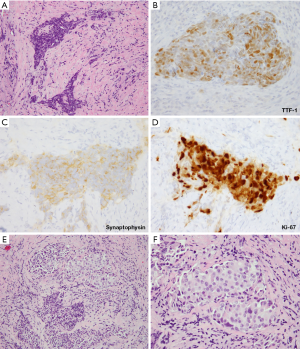
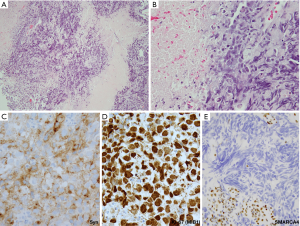
A new category of thoracic tumors has been recently described, which was initially designated as SMARCA4 (BRG1)-deficient thoracic sarcoma (83-85). Subsequent studies suggested that these tumors represent undifferentiated/dedifferentiated carcinomas (85,86). Histologically, these tumors are undifferentiated, discohesive and show high-grade round cell to rhabdoid morphology. Notably, these tumors commonly exhibit synaptophysin immunoreactivity (~70% of cases) (86). Morphologically, SD-UTT do not show NE architecture. However, they frequently show geographic necrosis and extremely high mitotic and Ki-67 rates, reflecting their highly aggressive nature. Thus, in a crushed biopsy, these features may closely mimic NE carcinomas. Clinical presentation can also be similar as these tumors present as large typically central masses in heavy smokers, although SD-UTT patients are frequently (but not always) younger than the patients with LCNEC or SCLC. Figure 9 illustrates one such case, in which initial diagnostic consideration was LCNEC. In addition to the loss of immunoreactivity for SMARCA4 (Figure 9E), a set of other IHC markers can be helpful in the diagnosis of SD-UTT and distinguishing these tumors from NE carcinomas. SD-UTT are characterized by the loss of epithelial differentiation, which can be demonstrated by the lack of claudin-4 (epithelial adhesion molecule) and low or absent keratin expression. Furthermore, SMARCA4 deficiency in SD-UTT is typically accompanied with co-deficiency for another SWI/SNF component SMARCA2 (BRM) and frequent expression of stem-cell markers (SALL4, CD34, and SOX2) (85,86).
Other entities in the differential diagnosis of LCNEC
In addition to the major entities discussed above, there is a number of other diagnostic considerations for tumors that enter in the differential diagnosis with LCNEC based on morphologic features and/or overlapping marker expression. In particular, metastases from the breast ductal carcinoma and prostate ADC may enter into consideration, as both of these tumor types can show prominent “endocrine” morphology (relatively monomorphic cytology and nested architecture). In addition, particularly in younger patients and/or never smokers, other rare monomorphic high-grade tumors with high N:C ratio can be encountered in the thoracic cavity and may mimic LCNEC, including NUT carcinoma, EBV-associated carcinoma [which may lack prominent lymphoid infiltrate typical of classic lymphoepithelioma-like carcinoma (87)], and round cell sarcomas (CIC-rearranged and even Ewing, which typically exhibits synaptophysin expression) among others. Thus, broader differential diagnosis and IHC workup may be needed in the context of clinicoradiologic findings.
Conclusions
The diagnosis of pulmonary LCNEC can present a major challenge owing to its morphologic and genomic heterogeneity, which overlaps with small cell and non-small cell lung carcinomas, and other entities. Although in recent years major progress has been made in understanding the molecular landscape of LCNEC, and data linking the molecular subsets to differences in clinical behavior are starting to emerge, translation of these findings to routine clinical practice awaits robust delineation of impact on patient management. Development of objective biomarkers to distinguish LCNEC from entities that enter into its differential diagnosis would be of great value, as would the development of specific biomarkers predictive of responses to individual systemic therapies.
Acknowledgments
The authors thank Dr. Joseph Montecalvo for help with the images in Figure 9.
Funding: This work was made possible in part by support from the National Cancer Institute Cancer Center Core Grant P30-CA008748 to Memorial Sloan Kettering Cancer Center.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.13). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 1991;15:529-53. [Crossref] [PubMed]
- Iyoda A, Hiroshima K, Toyozaki T, et al. Clinical characterization of pulmonary large cell neuroendocrine carcinoma and large cell carcinoma with neuroendocrine morphology. Cancer 2001;91:1992-2000. [Crossref] [PubMed]
- Takei H, Asamura H, Maeshima A, et al. Large cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases. J Thorac Cardiovasc Surg 2002;124:285-92. [Crossref] [PubMed]
- Kinslow CJ, May MS, Saqi A, et al. Large-cell neuroendocrine carcinoma of the lung: a population-based study. Clin Lung Cancer 2020;21:e99-e113. [Crossref] [PubMed]
- Asamura H, Kameya T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 2006;24:70-6. [Crossref] [PubMed]
- Naidoo J, Santos-Zabala ML, Iyriboz T, et al. Large cell neuroendocrine carcinoma of the lung: clinico-pathologic features, treatment, and outcomes. Clin Lung Cancer 2016;17:e121-9. [Crossref] [PubMed]
- Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke A, et al. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer, 2015.
- Viswanathan K, Siddiqui MT, Borczuk AC. Insulinoma-associated protein 1 is a sensitive and specific marker for lung neuroendocrine tumors in cytologic and surgical specimens. J Am Soc Cytopathol 2019;8:299-308. [Crossref] [PubMed]
- Doxtader EE, Mukhopadhyay S. Insulinoma-associated protein 1 is a sensitive and specific marker of neuroendocrine lung neoplasms in cytology specimens. Cancer Cytopathol 2018;126:243-52. [Crossref] [PubMed]
- Rooper LM, Sharma R, Li QK, et al. INSM1 Demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol 2017;41:1561-9. [Crossref] [PubMed]
- Pelosi G, Pasini F, Sonzogni A, et al. Prognostic implications of neuroendocrine differentiation and hormone production in patients with stage I nonsmall cell lung carcinoma. Cancer 2003;97:2487-97. [Crossref] [PubMed]
- Segawa Y, Takata S, Fujii M, et al. Immunohistochemical detection of neuroendocrine differentiation in non-small-cell lung cancer and its clinical implications. J Cancer Res Clin Oncol 2009;135:1055-9. [Crossref] [PubMed]
- Sterlacci W, Fiegl M, Hilbe W, et al. Clinical relevance of neuroendocrine differentiation in non-small cell lung cancer assessed by immunohistochemistry: a retrospective study on 405 surgically resected cases. Virchows Arch 2009;455:125-32. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG. Testing for neuroendocrine immunohistochemical markers should not be performed in poorly differentiated NSCCs in the absence of neuroendocrine morphologic features according to the 2015 WHO classification. J Thorac Oncol 2016;11:e26-7. [Crossref] [PubMed]
- Ye B, Cappel J, Findeis-Hosey J, et al. hASH1 is a specific immunohistochemical marker for lung neuroendocrine tumors. Hum Pathol 2016;48:142-7. [Crossref] [PubMed]
- Hiroshima K, Iyoda A, Shibuya K, et al. Prognostic significance of neuroendocrine differentiation in adenocarcinoma of the lung. Ann Thorac Surg 2002;73:1732-5. [Crossref] [PubMed]
- Linnoila RI, Mulshine JL, Steinberg SM, et al. Neuroendocrine differentiation in endocrine and nonendocrine lung carcinomas. Am J Clin Pathol 1988;90:641-52. [Crossref] [PubMed]
- Ionescu DN, Treaba D, Gilks CB, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation--an entity of no clinical or prognostic significance. Am J Surg Pathol 2007;31:26-32. [Crossref] [PubMed]
- Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res 2016;22:3618-29. [Crossref] [PubMed]
- Karlsson A, Brunnstrom H, Lindquist KE, et al. Mutational and gene fusion analyses of primary large cell and large cell neuroendocrine lung cancer. Oncotarget 2015;6:22028-37. [Crossref] [PubMed]
- Derks JL, Leblay N, Lantuejoul S, et al. New insights into the molecular characteristics of pulmonary carcinoids and large cell neuroendocrine carcinomas, and the impact on their clinical management. J Thorac Oncol 2018;13:752-66. [Crossref] [PubMed]
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun 2018;9:1048. [Crossref] [PubMed]
- Miyoshi T, Umemura S, Matsumura Y, et al. Genomic profiling of large-cell neuroendocrine carcinoma of the lung. Clin Cancer Res 2017;23:757-65. [Crossref] [PubMed]
- Derks JL, Leblay N, Thunnissen E, et al. Molecular subtypes of pulmonary large-cell neuroendocrine carcinoma predict chemotherapy treatment outcome. Clin Cancer Res 2018;24:33-42. [Crossref] [PubMed]
- Zhuo M, Guan Y, Yang X, et al. The prognostic and therapeutic role of genomic subtyping by sequencing tumor or cell-free DNA in pulmonary large-cell neuroendocrine carcinoma. Clin Cancer Res 2020;26:892-901. [PubMed]
- Glisson BS, Moran CA. Large-cell neuroendocrine carcinoma: controversies in diagnosis and treatment. J Natl Compr Canc Netw 2011;9:1122-9. [Crossref] [PubMed]
- Igawa S, Watanabe R, Ito I, et al. Comparison of chemotherapy for unresectable pulmonary high-grade non-small cell neuroendocrine carcinoma and small-cell lung cancer. Lung Cancer 2010;68:438-45. [Crossref] [PubMed]
- Sarkaria IS, Iyoda A, Roh MS, et al. Neoadjuvant and adjuvant chemotherapy in resected pulmonary large cell neuroendocrine carcinomas: a single institution experience. Ann Thorac Surg 2011;92:1180-6; discussion 1186-7. [Crossref] [PubMed]
- Shimada Y, Niho S, Ishii G, et al. Clinical features of unresectable high-grade lung neuroendocrine carcinoma diagnosed using biopsy specimens. Lung Cancer 2012;75:368-73. [Crossref] [PubMed]
- Varlotto JM, Medford-Davis LN, Recht A, et al. Should large cell neuroendocrine lung carcinoma be classified and treated as a small cell lung cancer or with other large cell carcinomas? J Thorac Oncol 2011;6:1050-8. [Crossref] [PubMed]
- Derks JL, van Suylen RJ, Thunnissen E, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinomas: does the regimen matter? Eur Respir J 2017. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Chauhan A, Arnold SM, Kolesar J, et al. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget 2018;9:14738-40. [Crossref] [PubMed]
- Mauclet C, Duplaquet F, Pirard L, et al. Complete tumor response of a locally advanced lung large-cell neuroendocrine carcinoma after palliative thoracic radiotherapy and immunotherapy with nivolumab. Lung Cancer 2019;128:53-6. [Crossref] [PubMed]
- Uprety D, Arjyal L, Vallatharasu Y, et al. Utilization of surgery and its impact on survival in patients with early stage small-cell lung cancer in the United States. Clin Lung Cancer 2020;21:186-193.e2. [Crossref] [PubMed]
- Raman V, Jawitz OK, Yang CJ, et al. Outcomes for surgery in large cell lung neuroendocrine cancer. J Thorac Oncol 2019;14:2143-51. [Crossref] [PubMed]
- Rekhtman N, Tafe LJ, Chaft JE, et al. Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod Pathol 2013;26:511-22. [Crossref] [PubMed]
- Rekhtman N, Travis WD. Large No More: The journey of pulmonary large cell carcinoma from common to rare entity. J Thorac Oncol 2019;14:1125-7. [Crossref] [PubMed]
- den Bakker MA, Willemsen S, Grunberg K, et al. Small cell carcinoma of the lung and large cell neuroendocrine carcinoma interobserver variability. Histopathology 2010;56:356-63. [Crossref] [PubMed]
- Ha SY, Han J, Kim WS, et al. Interobserver variability in diagnosing high-grade neuroendocrine carcinoma of the lung and comparing it with the morphometric analysis. Korean J Pathol 2012;46:42-7. [Crossref] [PubMed]
- Travis WD, Gal AA, Colby TV, et al. Reproducibility of neuroendocrine lung tumor classification. Hum Pathol 1998;29:272-9. [Crossref] [PubMed]
- Thunnissen E, Borczuk AC, Flieder DB, et al. The use of immunohistochemistry improves the diagnosis of small cell lung cancer and its differential diagnosis. an international reproducibility study in a demanding set of cases. J Thorac Oncol 2017;12:334-46. [Crossref] [PubMed]
- Azzopardi JG. Oat-cell carcinoma of the bronchus. J Pathol Bacteriol 1959;78:513-9. [Crossref] [PubMed]
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [Crossref] [PubMed]
- Marchevsky AM, Gal AA, Shah S, et al. Morphometry confirms the presence of considerable nuclear size overlap between "small cells" and "large cells" in high-grade pulmonary neuroendocrine neoplasms. Am J Clin Pathol 2001;116:466-72. [Crossref] [PubMed]
- Vollmer RT. The effect of cell size on the pathologic diagnosis of small and large cell carcinomas of the lung. Cancer 1982;50:1380-3. [Crossref] [PubMed]
- Hiroshima K, Iyoda A, Shida T, et al. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis. Mod Pathol 2006;19:1358-68. [Crossref] [PubMed]
- Fraire AE, Johnson EH, Yesner R, et al. Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol 1992;23:520-8. [Crossref] [PubMed]
- Travis WD. Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin 2014;24:257-66. [Crossref] [PubMed]
- Rekhtman N, Pietanza CM, Sabari J, et al. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod Pathol 2018;31:111-21. [Crossref] [PubMed]
- Baine MK, Sinard JH, Cai G, et al. A semiquantitative scoring system may allow biopsy diagnosis of pulmonary large cell neuroendocrine carcinoma. Am J Clin Pathol 2020;153:165-74. [Crossref] [PubMed]
- Nitadori J, Ishii G, Tsuta K, et al. Immunohistochemical differential diagnosis between large cell neuroendocrine carcinoma and small cell carcinoma by tissue microarray analysis with a large antibody panel. Am J Clin Pathol 2006;125:682-92. [Crossref] [PubMed]
- Lok BH, Gardner EE, Schneeberger VE, et al. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res 2017;23:523-35. [Crossref] [PubMed]
- Pietanza MC, Waqar SN, Krug LM, et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J Clin Oncol 2018;36:2386-94. [Crossref] [PubMed]
- Van Den Borg R, Leonetti A, Tiseo M, et al. Novel targeted strategies to overcome resistance in small-cell lung cancer: focus on PARP inhibitors and rovalpituzumab tesirine. Expert Rev Anticancer Ther 2019;19:461-71. [Crossref] [PubMed]
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology 2019;74:555-66. [Crossref] [PubMed]
- Howe MC, Chapman A, Kerr K, et al. Neuroendocrine differentiation in non-small cell lung cancer and its relation to prognosis and therapy. Histopathology 2005;46:195-201. [Crossref] [PubMed]
- Travis WD, Nicholson AG, Geisinger KR, et al. Large cell nueroendocrine carcinoma. Tumors of the lower respiratory tract. Arlington: American Registry of Path, 2019:vol 4.
- Pelosi G, Fabbri A, Bianchi F, et al. DeltaNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: a novel two-hit, sparing-material approach. J Thorac Oncol 2012;7:281-90. [Crossref] [PubMed]
- Bishop JA, Teruya-Feldstein J, Westra WH, et al. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 2012;25:405-15. [Crossref] [PubMed]
- Kadota K, Nitadori J, Rekhtman N, et al. Reevaluation and reclassification of resected lung carcinomas originally diagnosed as squamous cell carcinoma using immunohistochemical analysis. Am J Surg Pathol 2015;39:1170-80. [Crossref] [PubMed]
- Masai K, Tsuta K, Kawago M, et al. Expression of squamous cell carcinoma markers and adenocarcinoma markers in primary pulmonary neuroendocrine carcinomas. Appl Immunohistochem Mol Morphol 2013;21:292-7. [Crossref] [PubMed]
- Maleki Z. Diagnostic issues with cytopathologic interpretation of lung neoplasms displaying high-grade basaloid or neuroendocrine morphology. Diagn Cytopathol 2011;39:159-67. [PubMed]
- Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. [Crossref] [PubMed]
- Alcala N, Leblay N, Gabriel AAG, et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat Commun 2019;10:3407. [Crossref] [PubMed]
- Rekhtman N, Desmeules P, Litvak AM, et al. Stage IV lung carcinoids: spectrum and evolution of proliferation rate, focusing on variants with elevated proliferation indices. Mod Pathol 2019;32:1106-22. [Crossref] [PubMed]
- Aslan DL, Gulbahce HE, Pambuccian SE, et al. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol 2005;123:874-8. [Crossref] [PubMed]
- Lin O, Olgac S, Green I, et al. Immunohistochemical staining of cytologic smears with MIB-1 helps distinguish low-grade from high-grade neuroendocrine neoplasms. Am J Clin Pathol 2003;120:209-16. [Crossref] [PubMed]
- Pelosi G, Rindi G, Travis WD, et al. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 2014;9:273-84. [Crossref] [PubMed]
- Pelosi G, Rodriguez J, Viale G, et al. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005;29:179-87. [Crossref] [PubMed]
- Rindi G, Klersy C, Inzani F, et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer 2013;21:1-16. [Crossref] [PubMed]
- Walts AE, Ines D, Marchevsky AM. Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol 2012;25:1258-64. [Crossref] [PubMed]
- Warth A, Fink L, Fisseler-Eckhoff A, et al. Interobserver agreement of proliferation index (Ki-67) outperforms mitotic count in pulmonary carcinoids. Virchows Arch 2013;462:507-13. [Crossref] [PubMed]
- den Bakker MA, Thunnissen FB. Neuroendocrine tumours--challenges in the diagnosis and classification of pulmonary neuroendocrine tumours. J Clin Pathol 2013;66:862-9. [Crossref] [PubMed]
- Quinn AM, Chaturvedi A, Nonaka D. High-grade neuroendocrine carcinoma of the lung with carcinoid morphology: a study of 12 cases. Am J Surg Pathol 2017;41:263-70. [Crossref] [PubMed]
- Tsuta K, Raso MG, Kalhor N, et al. Histologic features of low- and intermediate-grade neuroendocrine carcinoma (typical and atypical carcinoid tumors) of the lung. Lung Cancer 2011;71:34-41. [Crossref] [PubMed]
- Huang Q, Muzitansky A, Mark EJ. Pulmonary neuroendocrine carcinomas. A review of 234 cases and a statistical analysis of 50 cases treated at one institution using a simple clinicopathologic classification. Arch Pathol Lab Med 2002;126:545-53. [PubMed]
- Uccella S, La Rosa S, Volante M, et al. Immunohistochemical biomarkers of gastrointestinal, pancreatic, pulmonary, and thymic neuroendocrine neoplasms. Endocr Pathol 2018;29:150-68. [Crossref] [PubMed]
- Tang LH, Basturk O, Sue JJ, et al. A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol 2016;40:1192-202. [Crossref] [PubMed]
- Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. [Crossref] [PubMed]
- Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 2015;47:1200-5. [Crossref] [PubMed]
- Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol 2017;30:1422-32. [Crossref] [PubMed]
- Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797-809. [Crossref] [PubMed]
- Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol 2020;15:231-47. [Crossref] [PubMed]
- Yeh YC, Kao HL, Lee KL, et al. Epstein-barr virus-associated pulmonary carcinoma: proposing an alternative term and expanding the histologic spectrum of lymphoepithelioma-like carcinoma of the lung. Am J Surg Pathol 2019;43:211-9. [Crossref] [PubMed]


