
Lung cancer occurrence attributable to passive smoking among never smokers in China: a systematic review and meta-analysis
Introduction
Environmental tobacco smoke is a common source of indoor air pollution worldwide (1,2), and its inhalation is known as passive smoking. Importantly, the International Agency for Research on Cancer has stated that passive smoking exposes people to the same carcinogens as active smoking, which is the leading cause of lung cancer (3). Consequently, passive smoking is considered an important cause of lung cancer in never smokers (3,4), increasing their risk of the disease (5). The biological plausibility for this association is that carcinogens and toxic substances seem to remain present in side-stream smoke and exhaled mainstream smoke (6-8).
Exposure to passive smoking continues to be a major public health concern, resulting in a large economic burden worldwide, including in China (1,9). Worldwide, it is estimated that 40% of children, 33% of males, and 35% of females identified as never smokers are exposed to passive smoking. The situation in China is complicated by having more tobacco consumers than any other country, with 316 million current smokers exposing more than 50% of never smokers to passive smoking in the home and workplace in 2015 (10). Depending on the study, estimates indicate that exposure to passive smoke in China varies from 34.1% to 72.4% (11-15). This wide range can be explained by variations in age and sex, as well as the region, source, and definition of exposure. Nevertheless, the large number of smokers necessitates that we quantify the effect of smoking on never smokers in the Chinese population to guide public health decisions.
In this systematic review, we aimed to estimate the proportion of lung cancers in never smokers that could be deemed attributable to passive smoking. To do so, we estimated the expected proportional reduction in lung cancer occurrence as if there had been no exposure to passive smoking, the so-called population attributable fraction (PAF) (16), assuming a causal relationship between passive smoking and lung cancer.
Methods
Data sources and search strategy
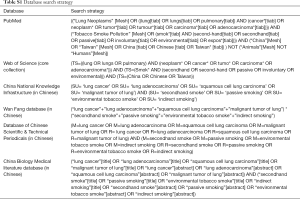
We conducted a comprehensive search of six databases for publications in English or Chinese in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (17). Articles published in English were identified through the PubMed and Web of Science databases. Those published in Chinese were found through the China National Knowledge Infrastructure, Database of Chinese Scientific & Technical Periodicals, Wan Fang database, and the China Biology Medical literature database.
All databases were searched from inception to July 2019 to identify original observational studies that reported relative risks (RRs) or odds ratios (ORs) of the association between passive smoking and lung cancer in Chinese never smokers. The following search terms were used: “tobacco smoke,” “secondhand smoking,” “passive smoking,” “lung cancer,” “China,” and “Chinese.” A detailed summary of the search strategy used in each database is described in Table S1. Additionally, we manually searched the reference lists of retrieved articles to identify relevant studies that were not revealed by the database search.
Full table
Eligibility criteria and study selection
Studies were included in the systematic review if they met the following criteria: participants were never smokers from China (including Taiwan), passive smoking was assessed at an individual level, risk estimates were reported for the occurrence of primary lung cancer, and a case-control or cohort design was used. Studies were excluded for the following reasons: if they focused on a specific occupational population (e.g., miners, catering workers, textile workers, oil field workers, or those exposed to asbestos or nuclear fuel); if they included residents of Xuanwei County of Yunnan Province [residents in this area have exceptionally high exposure to residential smoky coal emissions, which is associated with a 36-fold increase in lung cancer mortality in men and a 99-fold increase in women compared with smokeless coal (18)]; if the outcome of interest was the specific mortality instead of the occurrence of lung cancer; and if the proportion of primary lung cancer cases exposed to passive smoking was unavailable to calculate PAF. In the event of multiple publications from a single study, the most recent publication was selected.
Three reviewers independently screened the identified studies for inclusion. YD screened all studies, GS screened those published in English, and XC screened those published in Chinese. After a calibration session, any disagreement was mediated by a fourth reviewer (GHdB for the studies published in English and SL for the studies published in Chinese).
Data extraction and quality assessment
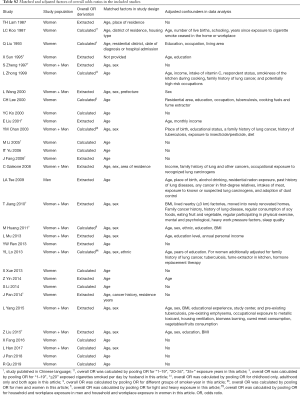
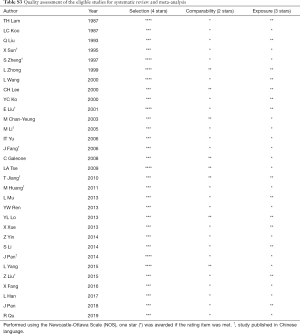
One author (Y Du) extracted data using a standardized extraction sheet (Figure S1) and two co-authors (G Sidorenkov, X Cui) reviewed the data. For each selected publication, three reviewers (Y Du, G Sidorenkov, X Cui) independently assessed the quality of included studies using the Newcastle-Ottawa Scale (NOS) (19). The NOS is a methodological assessment tool recommended for use with cohort and case-control studies that uses a star-based scale ranging from 0 to 9 stars (20). Quality is assessed on three domains in the NOS: (I) study group selection; (II) group comparability; and (III) exposure/outcome reliability. The comparability assessment needed to be further specified based on the topic of the analysis, which was done in a consensus meeting among the authors before assessing the studies. It was agreed that one star would be given when the comparison between cases and controls was adjusted for age and sex. Another star was given when there was adjustment for at least one of the following confounders: radon, asbestos, family history of lung cancer and cooking smoke. Any disagreements were settled by consensus or were adjudicated by a third reviewer (GHdB/SL). Studies assessed as zero points for the comparability domain were excluded from the meta-analysis.

Data analyses and syntheses
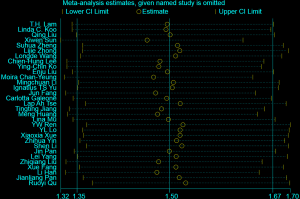
The first step involved a meta-analysis of the OR and corresponding 95% confidence intervals (CIs), using a random effects model. We performed I2 tests and considered data to have heterogeneity when the I2 value was >50%. For studies that reported both crude and adjusted OR estimates, the adjusted risk estimate was selected for the meta-analysis. For studies that reported stratified ORs, the overall OR was calculated by combing the stratified ORs and using them in the subgroup PAF calculations, as applicable. For studies that did not report OR directly, but where the necessary data were available, we performed the OR calculation ourselves. The derivation of the ORs used in the study, together with their matched/adjusted factors in each included study, are presented in Table S2. To evaluate the robustness of the pooled ORs, we performed sensitivity analyses in which each study was sequentially removed and the OR was recalculated. Publication bias was tested using Begg’s test and a funnel plot.
Full table
The next step involved calculating the point estimate of PAF based on the pooled proportion of exposed cases and the pooled OR (16,21), using the following formula:
where pc is the percentage of cases exposed in the combined population.
RR was replaced with the OR (as an approximation of the RR) for case-control studies (16). The 95% CI of the PAF was then estimated according to a formula described elsewhere, in which the variance of both the OR and the exposed cases were considered (21):
The variance of PAF is
The corresponding limits of ln(1-PAF) are
The upper limit (UL) and lower limit (LL) of PAF were calculated as 1-exp{LL[ln(1-PAF)]} and 1-exp{UL[ln(1-PAF)], respectively.
The meta-analysis was performed using Stata/SE software, version 15.0 (StataCorp., college Station, TX; package “pr0012”), and the PAF estimations were performed using Microsoft Excel 2010 (Microsoft Corporation, Washington).
Results
Eligible studies and their characteristics
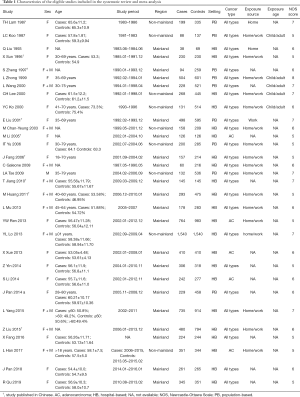
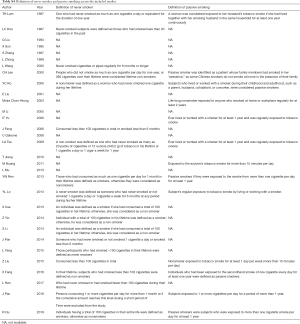
We identified 2,359 articles from the six databases we searched and retrieved 296 papers for full-text review; of these, 31 case-control studies [22 published in English (22-43) and 9 published in Chinese (44-52)] were eligible for inclusion (Figure 1). No cohort studies fulfilled the inclusion criteria. The details of all included studies are summarized in Table 1.
Full table
The average methodological quality score was 6.0±0.9, ranging from 5 to 8 (≥7 for 9 studies). Details of the quality assessment are presented in Table S3. Concerning exposure ascertainment, 29 studies had no blinding to the case/control status during interviews. Notably, the definitions of never smoker and passive smoking varied across the studies, as presented in Table S4.
Full table
Full table
Among the eligible studies, 9,614 cases of lung cancer and 13,093 controls were included, with exposure to passive smoking in 5,923 (61.6%) and 7,089 (54.1%), respectively. Overall, 11 studies included both men and women, 19 studies included only women, and 1 study included only men. The age of the population of interest in the included studies varied and was presented either as mean and standard deviation or percentage, as shown in Table 1. Most studies (n=22) were conducted in mainland China. The control groups were recruited from a hospital in 22 studies, but they were population-based in the remaining 9 studies. All but 5 studies, which were limited to lung adenocarcinoma, included all types of lung cancer. Of the 20 studies that provided data on the source of passive smoking, 18 considered both home and work exposure, 2 considered home exposure only, and 1 considered work exposure only.
The PAF for lung cancer due to passive smoking
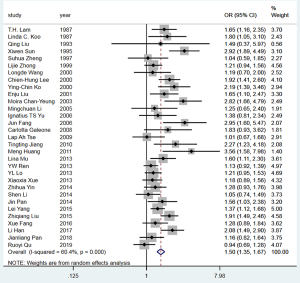
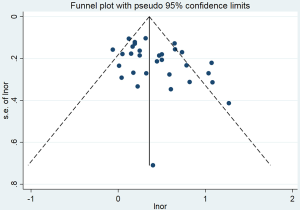
The pooled OR for lung cancer risk attributed to passive smoking in never smokers was 1.50 (95% CI: 1.35–1.67) (Figure 2), which was robust in the sensitivity analysis (Figure S2). However, heterogeneity was observed across the studies (I2=60.4%, P<0.001) and there was some evidence of publication bias according to Begg’s test (P=0.041) and an asymmetric funnel plot (Figure S3). The percentage of cases exposed to passive smoking was 61.6% (5,923/9,614), and the overall PAF for lung cancer due to passive smoking was 20.5% (95% CI: 15.9–24.9%).
The PAF for lung cancer due to passive smoking in population- and hospital-based studies
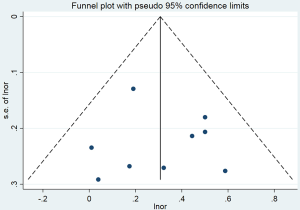
The pooled OR for passive smoking and lung cancer risk in never smokers was 1.36 (95% CI: 1.19–1.56) for the 9 population-based studies (Figure 3). Moreover, no heterogeneity was observed across the studies (I2=0%, P=0.537), and there was no publication bias, as indicated by Begg’s test (P=0.754) and a symmetrical funnel plot (Figure S4). In population-based studies, the PAF for lung cancer due to passive smoking was 15.5% (95% CI: 9.0–21.4%).
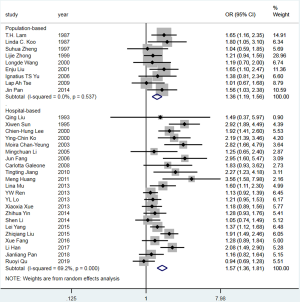
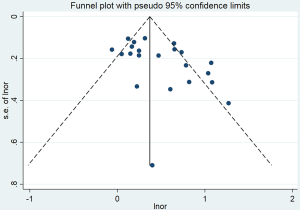
The pooled OR for passive smoking and lung cancer risk in never smokers was 1.57 (95% CI: 1.36–1.81) for the 22 hospital-based studies (Figure 3). However, substantial heterogeneity was observed (I2=69.2%, P<0.001), and there was some evidence of publication bias, as indicated by Begg’s test (P=0.048) and an asymmetrical funnel plot (Figure S5). In the hospital-based studies, the PAF for lung cancer due to passive smoking was 22.7% (95% CI: 16.6–28.3%) (Table 2).
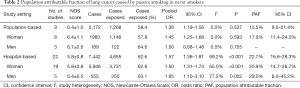
Full table
The PAF for lung cancer due to passive smoking in men and women
For the population-based studies, the pooled OR for passive smoking and lung cancer risk in female never smokers was 1.45 (95% CI: 1.25–1.68), with no heterogeneity (I2=0.0%, P=0.593) (Figure S6). The PAF for lung cancer due to passive smoking in this group was 17.9% (95% CI: 11.4–24.0%). The non-significant OR was yielded from the small number of population-based studies reporting the association between passive smoking and lung cancer risk in male never smokers meant that the PAF could not be estimated.
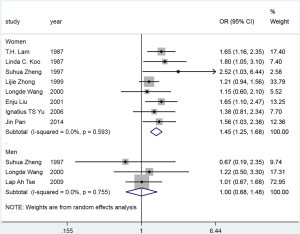
For the hospital-based studies, substantial heterogeneity was observed across studies (studies in females: I2=65.0%, P<0.001; studies in males: I2=77.2%, P=0.002) (Figure S7). The PAF for lung cancer due to passive smoking was 20.9% (95% CI: 14.7–26.7%) in females and 29.0% (95% CI: 8.0–45.2%) in males (Table 2).

The PAF for lung cancer due to passive smoking in women, based on exposure source
The pooled OR for passive smoking at home and lung cancer risk among female never smokers was 1.42 (95% CI: 1.21–1.67), with no significant heterogeneity (I2=40.8%, P=0.107) (Figure S8). The PAF for lung cancer due to passive smoking at home was 19.5% (95% CI: 11.4–26.9%). The pooled OR for passive smoking in the workplace and lung cancer risk among female never smokers was 1.58 (95% CI: 1.33–1.88), with no heterogeneity (I2=0.0%, P=0.962). The PAF for lung cancer due to passive smoking in the workplace was 7.2% (95% CI: 4.6–9.7%) (Table 3).
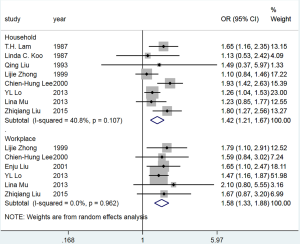
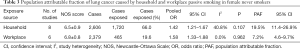
Full table
The PAF for lung cancer due to passive smoking by histological type
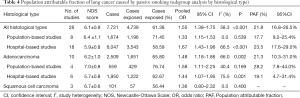
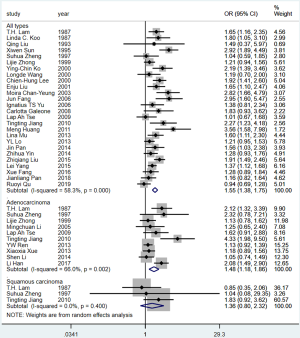
The pooled OR for passive smoking and lung adenocarcinoma risk from the population-based studies was 1.58 (95% CI: 1.11–2.25), with no significant heterogeneity across studies (I2=40.4%, P=0.169). The PAF for lung adenocarcinoma due to passive smoking was 28.2% (95% CI: 7.8–44.0%). PAF could not be estimated for the association between passive smoking and squamous cell carcinoma in never smokers because of the non-significant OR yielded from limited number of studies (Table 4, Figure S9).
Full table
Discussion
Main findings
We conducted a systematic review and meta-analysis based on evidence from nearly 23,000 participants in 31 studies. Our aim was to estimate the proportion of lung cancer cases that could be prevented by eliminating passive smoking in Chinese never smokers. Overall, using the PAF, we showed that approximately one-fifth of lung cancer cases were attributable to passive smoking, with a lower proportion from population-based studies (15.5%) than from hospital-based studies (22.7%). Given that population-based studies allow for more precise comparisons between cases and controls in a target population (53), data from these may have been more reliable (21). Furthermore, we demonstrated good homogeneity and no publication bias across the included population-based studies, indicating that the estimate from these data was unbiased. We conclude that the PAF estimate of 15.5% from population-based case-control studies was reliable. Regarding to the histological type of lung cancer, compared to the studies including all histological types, the proportion of lung adenocarcinoma caused by passive smoking in never smokers was higher (28.2% vs. 17.7%) based on the population-based studies.
The proportion of lung cancer cases that could be prevented among women by stopping passive smoking was 18% in this study, which was lower than the 24% reported in a previous estimate from 2008 (54). However, the RR of passive smoking for lung cancer was comparable with that in the previous publication, implying that there has been an overall decrease in the prevalence of passive smoking. This could be because China officially signed the Framework Convention on Tobacco Control in 2003 (55), which has resulted in several smoke-free policies being implemented (56-58). Additional positive effects on lung cancer occurrence can be expected from these measures because smoking rates decline slowly. The risk of lung cancer in exposed individuals may therefore decline further over time as exposure to passive smoking reduces.
The overall proportion of lung cancers attributable to passive smoking in Chinese never smokers (16%) was similar to that estimated for the United Kingdom (14–15%) in 2010 (59). However, it was much higher than that reported for the United States in 2014, where passive smoking contributed to only 2.7% of lung cancers (3.1% for men, 2.3% for women) in both never and ever smokers (60). The prevalence of smoking in the United States has decreased over several years (61), and it has been reported that the prevalence of passive smoking in nonsmokers was only 25.2% in 2014 (62). In the present study, the PAF for female never smokers for China (18%) was close to that estimated for Korea in 2009 (20.7%) (63) and Japan in 2005 (18.9%) (64). By contrast, in France, 6.7% of female lung cancers were attributable to domestic passive smoking, a rate that is much lower than reported for female never smokers in China (65). This could be due to the comparatively higher prevalence of passive smoking in China. Indeed, according to surveys in 2015, exposure to passive smoking in the home among female never smokers was 51.4% in China (10), whereas it was reported to range from 2.9% to 42.8% (increasing with age) in France (65).
The proportion of lung cancers attributable to passive smoking in the home (19.5%) was much higher than that in the workplace (7.2%) among women. The main reason for this appeared to be that more women were exposed to passive smoking in the home (66.0%) than in the workplace (19.6%). According to a survey of adults aged ≥40 years in China, 37.7% of never smokers exposed to passive smoking reported that they were usually exposed at home, whereas only 7.1% reported that they were usually exposed in the workplace (14). The home is therefore the predominant site of exposure to passive smoking, especially for women and children (12). One study indicated that this may reflect a displacement effect due to smoke-free legislation, with the net effect being that people smoke more frequently at home to avoid the restrictions in place at public places (66). As a priority, we therefore recommend that public health policy in China aim to reduce passive smoking in the home.
Limitations
Estimating the PAF in a systematic review and meta-analysis is an alternative approach when data on exposure rates are not available from national surveys. However, there are some limitations in the study. First, we used the OR from case-control studies as an approximation of the RR because there were no eligible cohort studies. Although this is not ideal, the OR from a case-control study is considered a valid substitute for the RR from a cohort study when a disease is uncommon (16). Second, we could not control for the effects of cooking fumes when estimating the PAF of lung cancer due to passive smoking in the home, which might have resulted in an overestimation of the PAF. Third, most of the studies had no blinding to the case/control status during interview, indicating a possible high risk of information or misclassification bias. Fourth, the PAF for male never smokers could not be estimated because there were insufficient population-based studies.
Conclusions
The results of this review and meta-analysis indicate that passive smoking contributes to about 16% of lung cancers in Chinese never smokers, but that this increases to 18% in females. Further measures are needed to control against the harmful effects of passive smoking, especially in Chinese women, and we recommend that public health efforts should prioritize reducing levels of passive smoking in the home. It appears that the biggest gains can be achieved here, not only by preventing lung cancer but also by preventing other diseases associated with passive smoking.
Acknowledgments
We thank Dr. Robert Sykes (www.doctored.org.uk) for providing editorial services.
Funding: Y Du is grateful for financial support from the China Scholarship Council (No. 201708340072). This work was supported by the Ministry of Science and Technology of the People’s Republic of China, National Key R&D Program of China (grant number: 2016YFE0103000), and the Royal Netherlands Academy of Arts and Sciences (grant number: PSA_SA_BD_01).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Öberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011;377:139-46. [Crossref] [PubMed]
- Zhang J, Smith KR. Indoor air pollution: a global health concern. Br Med Bull 2003;68:209-25. [Crossref] [PubMed]
- International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. IARC Monographs on the evaluation of carcinogenic risks to humans Lyon, France: IARC, 2004.
- Office of the Surgeon General (US). Publications and Reports of the Surgeon General. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US), 2006.
- Couraud S, Zalcman G, Milleron B, et al. Lung cancer in never smokers--a review. Eur J Cancer 2012;48:1299-311. [Crossref] [PubMed]
- Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009;15:5626-45. [Crossref] [PubMed]
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol 2007;25:561-70. [Crossref] [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [Crossref] [PubMed]
- Yao T, Sung HY, Mao Z, et al. The healthcare costs of secondhand smoke exposure in rural China. Tob Control 2015;24:e221-6. [Crossref] [PubMed]
- Chinese Center for Disease Control and Prevention. The 2015 China Adult Tobacco Survey. 2015.
- Xu Z, Han H, Zhuang C, et al. Tobacco Use and Exposure to Second-Hand Smoke among Urban Residents: A Community-Based Investigation. Int J Environ Res Public Health 2015;12:9799-808. [Crossref] [PubMed]
- Yang G, Fan L, Tan J, et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA 1999;282:1247-53. [Crossref] [PubMed]
- Wang CP, Ma SJ, Xu XF, et al. The prevalence of household second-hand smoke exposure and its correlated factors in six counties of China. Tob Control 2009;18:121-6. [Crossref] [PubMed]
- Cong S, Feng YJ, Bao HL, et al. Analysis on passive smoking exposure in adults aged 40 years and older in China, 2014. Zhonghua Liu Xing Bing Xue Za Zhi 2018;39:557-62. [PubMed]
- Xiao L, Yang Y, Li Q, et al. Population-based survey of secondhand smoke exposure in China. Biomed Environ Sci 2010;23:430-6. [Crossref] [PubMed]
- Mansournia MA, Altman DG. Population attributable fraction. BMJ 2018;360:k757. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Barone-Adesi F, Chapman RS, Silverman DT, et al. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ 2012;345:e5414. [Crossref] [PubMed]
- GA Wells BS, D O'Connell, J Peterson, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 29, 2019
- Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2-10. [Crossref] [PubMed]
- Steenland K, Armstrong B. An overview of methods for calculating the burden of disease due to specific risk factors. Epidemiology 2006;17:512-9. [Crossref] [PubMed]
- Koo LC, Ho JH, Saw D, et al. Measurements of passive smoking and estimates of lung cancer risk among non-smoking Chinese females. Int J Cancer 1987;39:162-9. [Crossref] [PubMed]
- Lam TH, Kung IT, Wong CM, et al. Smoking, passive smoking and histological types in lung cancer in Hong Kong Chinese women. Br J Cancer 1987;56:673-8. [Crossref] [PubMed]
- Liu Q, Sasco AJ, Riboli E, et al. Indoor air pollution and lung cancer in Guangzhou, People's Republic of China. Am J Epidemiol 1993;137:145-54. [Crossref] [PubMed]
- Zhong L, Goldberg MS, Gao YT, et al. A case-control study of lung cancer and environmental tobacco smoke among nonsmoking women living in Shanghai, China. Cancer Causes Control 1999;10:607-16. [Crossref] [PubMed]
- Ko YC, Cheng LS, Lee CH, et al. Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol 2000;151:140-7. [Crossref] [PubMed]
- Lee CH, Ko YC, Goggins W, et al. Lifetime environmental exposure to tobacco smoke and primary lung cancer of non-smoking Taiwanese women. Int J Epidemiol 2000;29:224-31. [Crossref] [PubMed]
- Wang L, Lubin JH, Zhang SR, et al. Lung cancer and environmental tobacco smoke in a non-industrial area of China. Int J Cancer 2000;88:139-45. [Crossref] [PubMed]
- Chan-Yeung M, Koo LC, Ho JC, et al. Risk factors associated with lung cancer in Hong Kong. Lung Cancer 2003;40:131-40. [Crossref] [PubMed]
- Yu IT, Chiu YL, Au JS, et al. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res 2006;66:4961-7. [Crossref] [PubMed]
- Galeone C, Pelucchi C, La Vecchia C, et al. Indoor air pollution from solid fuel use, chronic lung diseases and lung cancer in Harbin, Northeast China. Eur J Cancer Prev 2008;17:473-8. [Crossref] [PubMed]
- Tse LA, Yu IT, Au JS, et al. Environmental tobacco smoke and lung cancer among Chinese nonsmoking males: might adenocarcinoma be the culprit? Am J Epidemiol 2009;169:533-41. [Crossref] [PubMed]
- Lo YL, Hsiao CF, Chang GC, et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control 2013;24:567-76. [Crossref] [PubMed]
- Mu L, Liu L, Niu R, et al. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Causes Control 2013;24:439-50. [Crossref] [PubMed]
- Ren YW, Yin ZH, Wan Y, et al. P53 Arg72Pro and MDM2 SNP309 polymorphisms cooperate to increase lung adenocarcinoma risk in Chinese female non-smokers: a case control study. Asian Pac J Cancer Prev 2013;14:5415-20. [Crossref] [PubMed]
- Yang L, Lu X, Deng J, et al. Risk factors shared by COPD and lung cancer and mediation effect of COPD: two center case-control studies. Cancer Causes Control 2015;26:11-24. [Crossref] [PubMed]
- Fang X, Yin Z, Li X, et al. Polymorphisms in GEMIN4 and AGO1 Genes Are Associated with the Risk of Lung Cancer: A Case-Control Study in Chinese Female Non-Smokers. Int J Environ Res Public Health 2016;13:939. [Crossref] [PubMed]
- Han L, Lee CK, Pang H, et al. Genetic predisposition to lung adenocarcinoma among never-smoking Chinese with different epidermal growth factor receptor mutation status. Lung Cancer 2017;114:79-89. [Crossref] [PubMed]
- Pan JL, Gao J, Hou JH, et al. Interaction Between Environmental Risk Factors and Catechol-O-Methyltransferase (COMT) and X-Ray Repair Cross-Complementing Protein 1 (XRCC1) Gene Polymorphisms in Risk of Lung Cancer Among Non-Smoking Chinese Women: A Case-Control Study. Medical science monitor. Med Sci Monit 2018;24:5689-97. [Crossref] [PubMed]
- Xue X, Yin Z, Lu Y, et al. The Joint Effect of hOGG1, APE1, and ADPRT Polymorphisms and Cooking Oil Fumes on the Risk of Lung Adenocarcinoma in Chinese Non-Smoking Females. PLoS One 2013;8:e71157. [Crossref] [PubMed]
- Shen L, Yin Z, Wu W, et al. Single Nucleotide Polymorphism in ATM Gene, Cooking Oil Fumes and Lung Adenocarcinoma Susceptibility in Chinese Female Non-Smokers: A Case-Control Study. PLoS One 2014;9:e96911. [Crossref] [PubMed]
- Yin Z, Cui Z, Ren Y, et al. Genetic polymorphisms of TERT and CLPTM1L, cooking oil fume exposure, and risk of lung cancer: a case-control study in a Chinese non-smoking female population. Med Oncol 2014;31:114. [Crossref] [PubMed]
- Qu R, Li X, Quan X, et al. Polymorphism in CYP24A1 Is Associated with Lung Cancer Risk: A Case-Control Study in Chinese Female Nonsmokers. DNA Cell Biol 2019;38:243-9. [Crossref] [PubMed]
- Liu Z, He F, Cai L. A case-control study on smoking, passive smoking and the risk of lung cancer. Chinese Journal of Disease Control & Prevention 2015;19:145-9.
- Pan J, Gong WW, Wang H, et al. A case-control study on risk factors of lung cancer lung cancer among non-smoking women. Zhejiang Preventive Medicine 2014;26:772-4+82.
- Huang M, Chen X, Qiu YF, et al. Study on influencing factors and their interactions for lung cancer. Chinese Journal of Disease Control & Prevention 2011;15:91-4.
- Jiang T, Song H, Peng X, et al. A Case-control Study on Non-smoking Primary Lung Cancers in Sichuan, China. Zhongguo Fei Ai Za Zhi 2010;13:511-6. [PubMed]
- Fang J, Gan DK, Zheng SH, et al. A case-control study of the risk factors for lung cancer among Chinese women who have never smoked. Wei Sheng Yan Jiu 2006;35:464-7. [PubMed]
- Li M, Yin Z, Cui Z, et al. Association of genetic polymorphism in DNA repair gene XRCC1 with risk of lung adenocarcinoma in nonsmoking women. Zhongguo Fei Ai Za Zhi 2005;8:431-4. [PubMed]
- Liu EJ, Xiang YB, Jin F, et al. Risk factors for lung cancer among nonsmoking females in urban Shanghai: a population-based case-control study. Tumor 2001;21:421-5.
- Zheng S, Fan R, Wu Z. Studies on Relationship between Passive Smoking and Lung Cancer in Non-smoking Women. Zhonghua Yu Fang Yi Xue Za Zhi 1997;31:163-5. [PubMed]
- Sun XW, Lin CY, Dai XD, et al. Study on the association of environmental tobacco smoke and female lung cancer. Tumor 1995;15:185-96.
- Sedgwick P. Odds ratios II. BMJ 2010.c4971. [Crossref]
- Sisti J, Boffetta P. What proportion of lung cancer in never-smokers can be attributed to known risk factors? Int J Cancer 2012;131:265-75. [Crossref] [PubMed]
- World Health Organization. Parties to the WHO Framework Convention on Tobacco Control. Available online: http://www.who.int/fctc/signatories_parties/en/, Accessed October 29, 2019.
- Ministry of Health of China. Decisions on Completely Prohibiting Smoking in the National Health Care System from 2011. Beijing, China: 2009.
- Ministry of Education of the People's Republic of China and Ministry of Health of the People's Republic of China. Suggestion on Enhancing Tobacco Control Efforts in Schools. Beijing, 2010.
- Ministry of Health of the People’s Republic of China. Rules for the Implementation of Sanitation Administration Ordinance in Public Places. Beijing, 2011.
- Parkin DM. 2. Tobacco-attributable cancer burden in the UK in 2010. Br J Cancer 2011;105 Suppl 2:S6-S13. [Crossref] [PubMed]
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31-54. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults in the United States. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/. Accessed October 29, 2019
- Tsai J, Homa DM, Gentzke AS, et al. Exposure to Secondhand Smoke Among Nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep 2018;67:1342-6. [Crossref] [PubMed]
- Park S, Jee SH, Shin HR, et al. Attributable fraction of tobacco smoking on cancer using population-based nationwide cancer incidence and mortality data in Korea. BMC Cancer 2014;14:406. [Crossref] [PubMed]
- Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005--systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol 2012;23:1362-9. [Crossref] [PubMed]
- Cao B, Hill C, Bonaldi C, et al. Cancers attributable to tobacco smoking in France in 2015. Eur J Public Health 2018;28:707-12. [Crossref] [PubMed]
- Zheng ZL, Deng HY, Wu CP, et al. Secondhand smoke exposure of children at home and prevalence of parental smoking following implementation of the new tobacco control law in Macao. Public Health 2017;144:57-63. [Crossref] [PubMed]


