
Cystic brain metastases and RET fusion in lung cancer
Several worldwide societies endorse the baseline identification of druggable oncogenic alterations in advanced non-small cell lung cancers (NSCLC) as it harbors a meaningful impact in patients’ outcome. While the recommendations vary slightly, there is general consensus for baseline testing of EGFR and BRAF-mutations, along with ALK and ROS1 rearrangements, due to the benefit generated by either the FDA/EMA-approved targeted therapies with tyrosine kinase inhibitors (TKIs). Additional promising targeted therapies are available for genomic alterations such as RET and NTRK fusions, HER2 mutations and MET amplification or mutation. Nevertheless, neither all centers may assess these oncogenes at baseline, nor all centers may perform a next-generation sequencing (NGS) test for genomic profiling, despite its cost-effectiveness compared with sequential gene sequencing. Therefore, some specific and distinctive patients’ clinical characteristics could help physicians for requesting some of these additional genomic alterations in daily clinical practice.
Cystic aspects have been reported for ALK-rearranged NSCLC brain metastases, but the evolution towards the cystic morphology has been mainly observed after crizotinib treatment (1). Recently, two reports (2,3) have documented the strong correlation between RET fusion and the atypical features of cystic brain metastases in three advanced, treatment-naïve NSCLC patients after ruling out infectious origin. In an additional case, cystic brain metastases were evident in a background of leptomeningeal involvement responsive to the specific RET TKI, the LOXO-292 (4). In Figure 1, we report the previously unpublished MRI imaging of a RET-positive NSCLC patient who developed cystic brain metastases after having been treated for locally advanced disease.
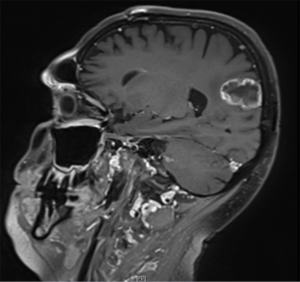
This observation has relevant clinical implications for assessing RET status in case it was not initially included in the upfront genomic portrait among patients with baseline cystic brain metastases. RET rearrangement is an uncommon targetable genomic alteration reported in up to 2% of NSCLC with KIF5B as the most common fusion partner gene. Brain metastases occur in 25% of advanced RET-rearranged NSCLC and selective RET tyrosine kinase inhibitors such as BLU-667 (5) in ARROW trial and LOXO292 (6) in LIBRETTO trial have reported clinically meaningful extracranial (response rate, RR: 60% and 68%, median progression free survival: not reached and 18.4 months, respectively) and intracranial efficacy (icRR: 78% and 91%, respectively) in RET-positive advanced NSCLC patients. This outcome mirrors the efficacy with personalised treatment reported in other druggable oncogenes in lung cancer, endorsing RET-fusions as a predictive biomarker for personalised treatment in NSCLC regardless the occurrence of brain metastases.
The identification of all the patients with a potential druggable genomic alteration is a priority in daily clinical practice. Based on this clinical data, the identification of baseline cystic brain metastases pattern should prompt, a quick test such as fluorescence in situ hybridization (in the lack of routine NGS), seeking for RET positivity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.12). JR reports other from MSD, other from BOEHRINGER, other from PFIZER, non-financial support and other from OSE IMMUNOTHERAPEUTICS, other from BMS, other from ASTRAZENECA, other from ROCHE, outside the submitted work; FF reports personal fees from Roche, BMS, outside the submitted work; BB reports grants from Abbvie, grants from Amgen, grants from AstraZeneca, grants from Biogen, grants from Blueprint Medicines, grants from BMS, grants from Celgène, grants from Eli-Lilly, grants from GSK, grants from Ignyta, grants from IPSEN, grants from Merck KGaA, grants from MSD, grants from Nektar, grants from Onxeo, grants from Pfizer, grants from Pharma Mar, grants from Sanofi, grants from Spectrum Pharmaceuticals, grants from Takeda, grants from Tiziana Pharma, outside the submitted work. JR serves as the unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. MT serves as the unpaid editorial board member of Translational Lung Cancer Research from Dec 2019 to Nov 2021.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saraceni C, Li PM, Gainor JF, et al. Cystic Brain Metastases in NSCLC Harboring the EML4-ALK Translocation after Treatment with Crizotinib. J Thorac Oncol 2015;10:1116-7. [Crossref] [PubMed]
- Facchinetti F, Bozzetti F, Gnetti L, et al. Wide and Cystic Brain Metastases Reveal RET-Rearranged Non–Small-Cell Lung Cancers. JCO Precis Oncol 2019. doi:. [Crossref]
- Remon J, Esteller L, Rodrigo MT, et al. Cystic Brain Metastases Revealed Patient With RET-Rearranged Non–Small-Cell Lung Cancer With Leptomeningeal Carcinomatosis and RET-Positive in CSF. JCO Precis Oncol 2020. doi:. [Crossref]
- Guo R, Schreyer M, Chang JC, et al. Response to Selective RET Inhibition With LOXO-292 in a Patient With RET Fusion-Positive Lung Cancer With Leptomeningeal Metastases. JCO Precis Oncol 2019;3. [Crossref] [PubMed]
- Gainor JF, Lee DH, Curigliano G, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:9008. [Crossref]
- Drilon A, Oxnard G, Wirth L, et al. Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. J Thorac Oncol 2019;14:S6-7. [Crossref]


