
Screening-detected pure ground-glass opacities: malignant potential beyond conventional belief?
It has been decades since the management of ground glass opacities (GGO) adopted a conservative approach. Nonetheless, recent new evidence has revealed the ‘malignant’ nature of lung lesions of GGO nature on computed tomography (CT) scan of the thorax. For pure GGO with pathological confirmation of malignant or premalignant behaviors, the chance of underlying frank adenocarcinoma could reach up to 30%, and the chance is even higher for part-solid GGOs (1-3). Interval CT monitoring is the most conservative management, but it is not surprising that 6-monthly or yearly CT scans usually cannot detect significant size progression of these slow-growing tumors, unless scans that are years apart were selected for comparison. After the announcement of the international multidisciplinary classification of lung adenocarcinoma (4), many follow-up studies unanimously suggested that the 5-year post-operative survival could reach 100% for adenocarcinoma in-situ and minimally-invasive adenocarcinoma, and excellent prognosis for lepidic-predominant adenocarcinoma (5). For clinically N0 lung cancer, incidence of lepidic-predominant adenocarcinoma for lesion size ≤10 mm was 97% for pure GGO lesion (6). Therefore, early surgical resection of GGOs, even for small or pure GGO lesions, not only offers diagnostic importance, but also offers significant survival benefit from early treatment (6). Recent advances in minimally-invasive thoracic surgery have enhanced the safety and efficacy of surgical management of subcentimeter or non-palpable GGO lesions, which were traditionally considered difficult to localize intraoperatively (7). The hybrid operation room allows real-time image guidance of localization tools, such as percutaneous hookwire insertion or pleural dye-marking via electromagnetic navigation bronchoscopy, for resection of small lung lesions (8). The combination of non-intubated anaesthesia, surgical techniques and instrumentations to minimize access trauma during uniportal video-assisted thoracic surgery (VATS) and enhanced recovery after surgery (ERAS) programs, further expanded the pool of surgical candidates of early diagnosis and treatment of these GGO lesions (9-11), and optimize postoperative recovery. Furthermore, for select non-surgical candidates, newer treatment modalities such as navigation bronchoscopy guided microwave ablation could provide good local control for small GGOs, with minimal procedural morbidity (12).
Robbins et al. in their recent report extracting pure GGOs from the National Lung Screening Trial database in 2011, highlighted some important consideration of management of pure GGOs detected on screening CT (13). Under the same Lung Computed Tomographic Screening Reporting and Data System (Lung-RADS) categorization, the malignancy probabilities of GGOs were in fact higher than that of solid nodules in the same category. For example, for solid nodules 6–7 mm being classified into Lung-RADS 3 (with malignancy probability of 1%), GGOs of 6–7 mm (carrying malignancy probability of 1.1%) were categorized into Lung-RADS 2, which erroneously suggested a lower malignancy probability. This under-estimation of the malignant potential of GGOs could lead to inadequately long intervals between follow-up scans or higher threshold for diagnostic and surgical interventions. Although this study included only pure GGOs and excluded GGOs with solid components, the same awareness should be escalated for GGOs with whatever proportion of solid component. In fact, rarely we have found even small GGOs to be clinically very aggressive and that can metastasize early (Figure 1).
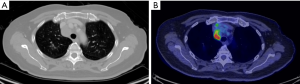
In face of the significant malignant potential of GGOs, the more accurate and less risky surgical approach to resection of these GGOs, and novel alternate therapeutic options such as bronchoscopic ablation, it may be worthwhile to push the current guidelines towards a more aggressive approach towards diagnosis and treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hattori A, Matsunaga T, Takamochi K, et al. Importance of Ground Glass Opacity Component in Clinical Stage IA Radiologic Invasive Lung Cancer. Ann Thorac Surg 2017;104:313-20. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Neither Maximum Tumor Size nor Solid Component Size Is Prognostic in Part-Solid Lung Cancer: Impact of Tumor Size Should Be Applied Exclusively to Solid Lung Cancer. Ann Thorac Surg 2016;102:407-15. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically "solid" tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Hayashi T, et al. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:954-62. [Crossref] [PubMed]
- Zhao ZR, Lau RWH, Ng CSH. Hybrid Theater and Uniportal Video-Assisted Thoracic Surgery: The Perfect Match for Lung Nodule Localization. Thorac Surg Clin 2017;27:347-55. [Crossref] [PubMed]
- Ng CSH, Chu CM, Lo CK, et al. Hybrid operating room Dyna-computed tomography combined image-guided electromagnetic navigation bronchoscopy dye marking and hookwire localization video-assisted thoracic surgery metastasectomy. Interact Cardiovasc Thorac Surg 2018;26:338-40. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2014;147:1095-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Gao S, Barello S, Chen L, et al. Clinical guidelines on perioperative management strategies for enhanced recovery after lung surgery. Transl Lung Cancer Res 2019;8:1174-87. [Crossref] [PubMed]
- Chan JWY, Yu PSY, Lau RWH, et al. Hybrid operating room—one stop for diagnosis, staging and treatment of early stage NSCLC. J Thorac Dis 2020;12:123-31. [Crossref] [PubMed]
- Robbins HA, Katki HA, Cheung LC, et al. Insights for Management of Ground-Glass Opacities From the National Lung Screening Trial. J Thorac Oncol 2019;14:1662-5. [Crossref] [PubMed]


