
Selected highlights of the 2019 Pulmonary Pathology Society Biennial Meeting: PD-L1 test harmonization studies
Introduction
Immune checkpoint inhibitors (ICI) which target the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) axis to restore antitumor immunity are now available in routine clinical practice for the treatment of both non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) patients with advanced or metastatic disease. For nearly 20% of NSCLC patients treated with ICIs a clear benefit has been reported, with increased progression-free survival (PFS) and/or overall survival (OS) (1-4). ICI are available as single immunotherapy agents, as well as in combination with chemotherapy. Pembrolizumab alone has been approved by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and Japanese Ministry of Health, Labour and Welfare (MHLW) in first-line treatment: (I) of metastatic NSCLC patients selected on the expression of PD-L1 by 50% or more of tumor cells (TCs) (which corresponds to a Tumor Proportion Score or TPS ≥50%) by immunohistochemistry (IHC), and in the absence of EGFR/ALK alterations (1,4); (II) in stage III wild-type EGFR/ALK patients who are not candidates for surgical resection or definitive chemoradiation and with a NSCLC with a TPS ≥1% (5). Regarding association of ICI and chemotherapy in first line setting, pembrolizumab was approved in combination with platinum/pemetrexed chemotherapy in non-squamous NSCLC, and with platinum/paclitaxel or nab-paclitaxel chemotherapy in squamous cell NSCLC (2,3). Atezolizumab has been authorized by the FDA in combination with platinum-paclitaxel-bevacizumab chemotherapy for non-squamous NSCLC with no EGFR/ALK alterations, and in Europe for patients with EGFR mutations or ALK rearrangements after tyrosine kinase inhibitors (6,7). In NSCLC patients previously treated with platinum-based chemotherapy, nivolumab and atezolizumab have been approved respectively by the FDA, EMA, and MHLW independently of PD-L1 expression by the TCs, and pembrolizumab when tumor exhibits a TPS ≥1% (8-10). Durvalumab has also been endorsed as consolidation treatment for unresectable, locally-advanced stage III NSCLC with no disease progression after chemoradiotherapy with a restriction to PD-L1 positive tumors (TPS≥1%) in Europe and Japan (11,12). Noteworthy, some immunotherapies are now available to SCLC patients in first, third- or later-line with single agent immunotherapy or in first line in combination with chemotherapy (13,14).
Several biomarkers have been reported to predict tumor response, but to date only PD-L1 expression assessed by IHC has been validated as a companion or complementary diagnostic to select patients who are more likely to take a real advantage from those therapies. It remains a semi-quantitative test that can be interpreted according to different scores, the most commonly used being TPS, or cut-off for PD-L1 expression, which opens eligibility for several indications of anti-PD-1 or anti-PD-L1 treatments.
To date, four PD-L1 IHC assays have been validated in clinical trials for the administration of the corresponding agents. Nevertheless, many pathology laboratories have set up laboratory-developed tests, which are less expensive than the clinical trial-validated assays and do not require a dedicated platform. In addition, the small size of most lung cancer samples precludes testing each sample with different assays. Although PD-L1 IHC testing is now implemented in most pathology laboratories, harmonization and validation of the protocols is still required to facilitate the appropriate implementation of this test, which is to date the only one offering a predictive value for anti-PD-(L)1 agents in the clinical setting. The objective herein is to provide an overview of the different assays available either as companion or complementary diagnostics for ICI, and to discuss the main comparative studies between LDT and assays (15-17).
Validated assays
To date, four assays have been clinically confirmed in randomized trials for specific anti-PD-1 or anti-PD-L1 agents in NSCLC. They have been approved either as companion or complementary tests, a companion diagnostic test being required for the prescription of a given therapy, whereas a complementary test is not required but can be helpful to select patients who could benefit from the treatment. Those assays are all based on a semi-quantitative assessment of PD-L1 expression on tumor tissue fixed in formalin and embedded in paraffin (FFPE) by IHC, but they use different primary monoclonal antibodies, platforms, detection systems, and scoring systems with various positivity thresholds validated in clinical trials. The PD-L1 IHC 22C3 PharmDx includes a mouse anti-PD-L1 clone 22C3. It was approved by the FDA and the MHLW and received CE-IVD (Conformité Européenne marking for In Vitro Diagnostic) designation in Europe to be used as a companion diagnostic test for the prescription of pembrolizumab (18). The PD-L1 IHC 28-8 PharmDx uses a rabbit anti-PD-L1 28-8 clone and has been endorsed by the FDA as a complementary diagnostic for the prescription of nivolumab. Both must be performed on the Agilent/Dako Autostainer Link 48 (Agilent Technologies, Santa Clara, CA, USA) with the EnVision FLEX visualization system, and at least 100 viable TCs are required for their interpretation (19). The Ventana PD-L1 (SP263) assay has obtained CE-IVD designation in Europe for durvalumab, pembrolizumab and nivolumab in NSCLC. This assay includes a rabbit anti-PD-L1 clone and has to be performed on Ventana BenchMark Ultra platform (Ventana Medical Systems, Tucson, AZ, USA). For PD-L1 IHC 22C3 PharmDx, PD-L1 IHC 28-8 PharmDx and the Ventana PD-L1 (SP263) assay, the score to be used is the Tumor Proportion Score (TPS), corresponding to the percentage of viable TCs exhibiting a partial or complete membranous staining at any intensity. The test is considered as positive if TPS ≥1%, with a high PD-L1 expression when TPS ≥50% (20). The EMA and the FDA have approved the PD-L1 SP142 (Ventana) assay as a complementary test for prescription of atezolizumab. This assay includes a rabbit anti-PD-L1 SP142 clone and must be performed on Ventana BenchMark Ultra platform. The scoring system used for this assay differs from the previous ones and takes into account the proportion of viable TC showing PD-L1 membrane staining of any intensity, but if TPS <50%, it considers also the proportion of tumor area occupied by immune cells (IC) with PD-L1 expression of any intensity. The scores retained for atezolizumab treatment are either TC3 (TC ≥50%) or IC3 (IC ≥10%) (21,22).
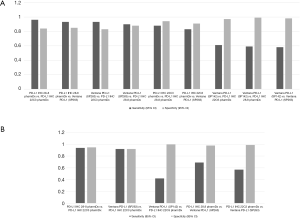
Those assays have been approved for a given drug on a given platform, but not all laboratories are equipped with multiple platforms, questioning the interchangeability of these assays in clinical practice. Regarding the clones themselves, Gaule et al. found that SP142, E1L3N, 9A11, SP263, 22C3, and 28-8 were high concordant when the same protocol was used to stain PD-L1 and concluded that the discordance reported between the assays was probably related to tumor heterogeneity or assay- or platform-specific variables (23). When the assays were compared, the 22C3 PharmDX, the 28-8 PharmDX and the Ventana PD-L1 SP263 assays have been reported to offer similar analytical performance for the tumor cells staining (16,24-29) with moderate to good kappa weighted ranging from 0.63 to 0.89. In contrast, other series showed a higher PD-L1 staining of the TC by the SP263 assay (30-33). A similar prevalence of PD-L1 expression was observed with 22C3 and 28-8 assays at 1% and 50% cut-off (34), with a strong correlation (OPA 97–98%) or a high weighted kappa across all samples (30-33,35,36). Other studies have focused their comparison between the 22C3 PharmDX and the Ventana PD-L1 SP263 assays, which have been both validated for the pembrolizumab prescription as well as for durvalumab treatment, even if their interchangeability in the clinical setting has not been validated on a large scale (37). Whereas Fujimoto et al. found no differences between those two assays with agreement rates of the 22C3 and SP263 assays of 88% to 97% at various cut-offs (37), Kim et al. observed similar TPS at low cut-offs but higher TPS with SP263 at high cut-offs (≥10%), and Munari et al. higher TPS with SP263 at both 1% and 50% cut-offs (38,39). The reliability and interchangeability of the assays on small samples were addressed by two studies, the Blueprint phase 2B project (32) and the study conducted by Kim et al. (40). Both evaluated the PD-L1 expression heterogeneity on matched specimen of lung cancer including surgical resection, core needle biopsy and fine needle aspiration cytology. A good agreement among pathologists was observed in assessing PD-L1 status on cell blocks in both studies, with an ICC from 0.78 to 0.85 for the first study, and a kappa coefficient for agreement around 0.65 at 1% cut off for 22C3 and 0.58 at 50% cut off for SP263 for the second. Of note, most studies agreed on the low TPS found on TC with the SP142 assay (24,30-33,41) and the poor agreement for the IC assessment between observers with all the assays used (27,30,42). Recently a meta-analysis of 22 publications including 376 assay comparisons at different cut-off points, has addressed PD-L1 assay interchangeability based on diagnostic accuracy, sensitivity and specificity of the tests for established clinical purposes (43). Considering that a test was adequate for clinical applications when both sensitivity and specificity were ≥90%, the authors found that PD-L1 IHC 28.8 pharmDx and Ventana PD-L1 (SP263) showed a precision sufficient for diagnostic at 50% cut-off, with a lower specificity at 1%. In contrast, PD-L1 IHC 22C3 pharmDx did not attain ≥90% for both sensitivity and specificity at 1% cut off. When SP263 was considered as the reference test, most other assays reached an acceptable specificity, even if the sensitivity was too low for both 1% and 50% cut-off points (Table 1; Figure 1A,B).
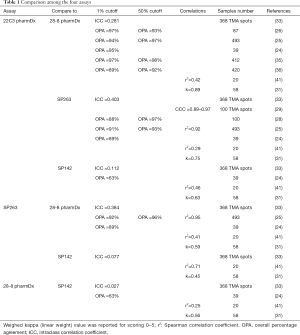
Full table
Laboratory developed tests (LDT)
Laboratories may, by choice or necessity, implement an IHC PD-L1 test that has not been validated in a clinical trial. The main factors explaining the use of LDT for PD-L1 testing are the unavailability of IHC platforms to perform dedicated assays (in particular Agilent/Dako AS Link 48 to perform the 22C3 companion diagnostic assay for pembrolizumab) and the lack of funding (either public or industry-sponsored) given the high cost of reagents to perform standardized assays. An LDT differs from a clinically-validated and standardized assay by its reagents (primary clone, detection kits), the platform used or the protocol integrating different methods of antigen retrieving, detection or amplification. These tests have been frequently developed with the concentrated antibodies used in the assays (clones 22C3 and 28-8) or with other monoclonal antibodies not used in the assays, in particular the clone E1L3N (validated for research use only - RUO) or the clone QR1 (CE-IVD labeled) (44). More rarely, LDT have been set up with pre-diluted antibodies recovered from PD-L1 assays, to be used on non-dedicated platforms such as Leica Bond IHC platforms.
Given the abundant literature on the topic for the last years, some LDT can reasonably provide technical performance comparable to that of tests validated. Many studies have proposed LDTs combining different clones, platforms and protocols with performance comparable to the standards validated in clinical trials (45-47), but most of these studies were based on a single center experience. The NCCN multicenter study (42) has shown that an LDT using the E1L3N clone could give satisfactory results, but after having thoroughly validated the different protocols. Two other multicenter studies conducted by the French thoracic pathology group PATTERN (27) and in Germany (30) have evaluated the used of several clones on several IHC platforms. These studies have shown very similar results with approximately one half of LDT evaluated (14/27 and 6/11, respectively) considered as concordant enough for clinical use, emphasizing the difficulty in validating PD-L1 LDT. Some combinations of antibodies and platforms were also identified as most effective in achieving a good concordance with dedicated PD-L1 assays (27). Recently, a Swiss cross-validation study has shown that clone 22C3 provided satisfying results on Ventana Benchmark Ultra platform, but a high variability on Leica Bond platforms (48). A summary of all the studies ‘results’ assessing inter-assay, inter-laboratory and inter-observer concordance among standardized assays and LDT has been recently published (49). A recent meta-analysis (43) has demonstrated that the highest diagnostic accuracy was observed between the 22C3 LDT and the PD-L1 IHC pharmDx 22C3, with sensitivity and specificity of 100% in 8 out of 9 assays for the 50% cut-off and almost at the 1% cut-off, in contrast with E1L3N which showed excellent results, but in 3 out of 12 comparisons. The authors noted that a properly validated LDT may have a higher diagnostic accuracy than PD-L1 FDA-endorsed companion diagnostic assay, and suggest that when a laboratory is unable to use a test that has been validated and approved in clinical practice for a given treatment, it is preferable to develop a LDT and to rigorously validate it in comparison with the recommended assay, rather than using another assay validated for another indication. It should be noted that these concordance studies may be limited by many factors such as sample types, positivity levels and pathologists’ intra-observer and inter-observer variability (29,46,47,50-52). Thus, the use of LDT has to be validated properly in each center. The main data are summarized in Table 2.
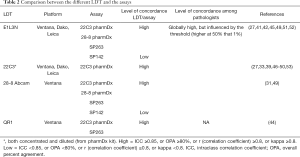
Full table
Practical considerations
PD-L1 stays an imperfect biomarker, as it is both a dynamic and an inducible marker with biological variations of expression according to histological sub-types, previous treatments and many biological factors (production of interferon gamma, STING inhibition, oncogene-driven expression, LKB1/STK11, KEAP1 or P53 mutations, amplification of PD-L1, Hypoxia, Epithelial-Mesenchymal Transformation, epigenetic regulation, etc.) (54-60).
In addition, there is a certain degree of inter-tumoral heterogeneity of PD-L1 expression during tumor progression, with some variations between primary and metastatic tissues (15). PD-L1 intra-tumoral heterogeneity is also an issue (61) and dictates a limit in the reliability of small samples. It has been recently shown in a cross-validation using TMA versus whole section scores by the European Thoracic Oncology Platform (ETOP) pathologists that PD-L1 status on small biopsies did not totally represent the overall expression of PD-L1 on surgical samples (62). Actually, the ideal would be to perform more biopsies (ideally 4 cores) with larger samples containing more than 2,000 tumor cells (62-65). Interestingly, they showed that the frequency of cases presenting a major discrepancy (i.e., with one core with a TPS <1% and another with a TPS >50%) did not exceed 2.1%. Moreover, they observed a tendency to underestimate the expression of PD-L1 on small samples, which may explain the good responses of patients with low levels or no PD-L1 expression in some trials.
Whatever, it is essential that the test used in clinical practice is the most accurate in order to comply with the indications validated by a trial and approved by the medical authorities for patient selection. The simplest way is to use the protocol validated by the clinical trial associated with the planned therapy and standardize all the pre-analytical steps, such as fixation and tissue processing and sectioning that may influence the immunohistochemical results.
Of note, PD-L1 expression is a continuous variable and there is no to date any method to rigorously evaluate the analytical sensitivity and specificity of a given PD-L1 IHC assay; however, it is very important to try to implement as rigorously as possible both trial validated assays and LDT in routine clinical practice. Interestingly, different protocols of LDTs with the 22C3 clone used on either Ventana BenchMark Ultra, Bond III (Leica Biosystems) or DAKO Omnis autostainers are available to date (46,47,50) and offer 85–100% agreement with the 22C3 PharmDx test on 48 Link Dako/Agilent autostainer. There is also a number of recommendations for the implementation of a theranostic test that have been proposed by the College of American Pathologists (CAP) (66-69). They recommend comparing each new test with expected results (clinically and morphologically), with the results obtained in another laboratory on the same samples, and with the results obtained using previously validated assay. One suggestion for the development of a protocol is to use a set of at least 20 PD-L1 positive and 20 negative cases tested with a reference assay. Ideally, this set should be expanded on cases close to the clinically relevant positivity thresholds, currently ≥1% and ≥50% of tumor cells with membranous staining (62,70). In addition, it is essential to monitor the ongoing performance of the test by checking continuously its sensitivity and specificity, the inter-run and inter operator variability, and the positive and negative concordance rates, a minimal 90% overall concordance rate (corresponding approximately to a kappa value ≥0.75) as compared to the reference test being expected. In each run, since an inter-slide variability may be observed, the use of an external positive control (such as tonsil tissue containing epithelial and IC staining or cell lines) on each slide is suggested. Finally, participation to External Quality Assurance programs is crucial as well as continuous monitoring of positivity rates for clinically relevant thresholds.
Conclusions
PD-L1 expression in tumor cells is recognized as a predictive biomarker for the first- and second-line prescription of ICI in advanced stage NSCLC. This expression is assessed using either clinically validated assays or LDT set up in most laboratory tests along with other theranostic biomarkers. However, given the imperfect interchangeability of those tests, they have to be validated before their implementation in clinical setting and to be monitored regularly in agreement with specific recommendations regarding pre-analytical, analytical and post-analytical steps. Another major point to be awarded of is the interobserver variability of PD-L1 assessment in particularly for the 1% cut-off, as pointed out in several studies (27,30,42,53,71,72). Interobserver discrepancies tend to be higher when a multistep scoring system is used and lower for individual cut-off values. The most recent studies showed a higher concordance among the pathologists, suggesting that the increasing level of experience of pathologists in PD-L1 IHC interpretation can help in getting more reliable PD-L1 assessment. In addition to IHC staining validation, training of pathologists is thus required to increase the reproducibility of PD-L1 assessment by pathologists (31,71) and ensure reliability of PD-L1 testing in NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.23). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. SL reports personal fees from Astra Zeneca, personal fees from MSD, grants and personal fees from BMS, during the conduct of the study; personal fees from Abbvie, outside the submitted work; JA reports personal fees from BMS, grants and personal fees from MSD, personal fees from AstraZeneca, personal fees from Roche, during the conduct of the study; FD has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Reck M, Shankar G, Lee A, et al. Atezolizumab in combination with bevacizumab, paclitaxel and carboplatin for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer, including patients with EGFR mutations. Expert Rev Respir Med 2020;14:125-36. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, von Pawel J, Park K, et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1156-70. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Peters S, Dafni U, Boyer M, et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann Oncol 2019;30:161-5. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic Small-Cell Lung Cancer: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol 2019. [Epub ahead of print].
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective from the IASLC Pathology Committee. J Thorac Oncol 2020;15:499-519. [Crossref] [PubMed]
- Lantuejoul S, Damotte D, Hofman V, et al. Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J Thorac Dis 2019;11:S89-101. [Crossref] [PubMed]
- Available online: 22C3https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdfmanuel
- Available online: https://www.agilent.com/cs/library/usermanuals/public/29111_pd-l1-ihc-28-8-interpretation-manual.pdf
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160046c.pdf
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160006C.pdf
- Spigel DR, Chaft JE, Gettinger S, et al. FIR: Efficacy, Safety, and Biomarker Analysis of a Phase II Open-Label Study of Atezolizumab in PD-L1-Selected Patients With NSCLC. J Thorac Oncol 2018;13:1733-42. [Crossref] [PubMed]
- Gaule P, Smithy JW, Toki M, et al. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol 2017;3:256-9. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:3585-91. [Crossref] [PubMed]
- Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx Appl Immunohistochem Mol Morphol 2017;25:453-9. [Crossref] [PubMed]
- Adam J, Le Stang N, Rouquette I, et al. Multicenter French harmonization study for PD-L1 IHC testing in non-small cell lung cancer. Ann Oncol [Internet]. 16 janv 2018 [cité 18 janv 2018]. Available online: http://academic.oup.com/annonc/advance-article/doi/10.1093/annonc/mdy014/4812668
- Fujimoto D, Yamashita D, Fukuoka J, et al. Comparison of PD-L1 Assays in Non-small Cell Lung Cancer: 22C3 pharmDx and SP263. Anticancer Res 2018;38:6891-5. [Crossref] [PubMed]
- Marchetti A, Barberis M, Franco R, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol 2017;12:1654-63. [Crossref] [PubMed]
- Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 2016;29:1165-72. [Crossref] [PubMed]
- Brunnström H, Johansson A, Westbom-Fremer S, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod Pathol 2017;30:1411-21. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]
- Hendry S, Byrne DJ, Wright GM, et al. Comparison of Four PD-L1 Immunohistochemical Assays in Lung Cancer. J Thorac Oncol 2018;13:367-76. [Crossref] [PubMed]
- Velcheti V, Patwardhan PD, Liu FX, et al. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PloS One 2018;13:e0206370. [Crossref] [PubMed]
- Batenchuk C, Albitar M, Zerba K, et al. A real-world, comparative study of FDA-approved diagnostic assays PD-L1 IHC 28-8 and 22C3 in lung cancer and other malignancies. J Clin Pathol 2018;71:1078-83. [Crossref] [PubMed]
- Saito T, Tsuta K, Ishida M, et al. Comparative study of programmed cell death ligand-1 immunohistochemistry assays using 22C3 and 28-8 antibodies for non-small cell lung cancer: Analysis of 420 surgical specimens from Japanese patients. Lung Cancer 2018;125:230-7. [Crossref] [PubMed]
- Fujimoto D, Sato Y, Uehara K, et al. Predictive Performance of Four Programmed Cell Death Ligand 1 Assay Systems on Nivolumab Response in Previously Treated Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:377-86. [Crossref] [PubMed]
- Kim HR, Cha YJ, Hong MH, et al. Concordance of programmed death-ligand 1 expression between primary and metastatic non-small cell lung cancer by immunohistochemistry and RNA in situ hybridization. Oncotarget [Internet]. Available online: http://www.oncotarget.com/fulltext/20254
- Munari E, Rossi G, Zamboni G, et al. PD-L1 Assays 22C3 and SP263 are Not Interchangeable in Non-Small Cell Lung Cancer When Considering Clinically Relevant Cutoffs: An Interclone Evaluation by Differently Trained Pathologists. Am J Surg Pathol 2018;42:1384-9. [Crossref] [PubMed]
- Kim I, Kim A, Lee CH, et al. Reliability of PD-L1 assays using small tissue samples compared with surgical specimens. Medicine (Baltimore) 2019;98:e14972. [Crossref] [PubMed]
- Soo RA, Yun Lim JS, Asuncion BR, et al. Determinants of variability of five programmed death ligand-1 immunohistochemistry assays in non-small cell lung cancer samples. Oncotarget 2018;9:6841-51. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Torlakovic E, Lim HJ, Adam J, et al. «Interchangeability» of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol 2020;33:4-17. [Crossref] [PubMed]
- Brandone N, Mascaux C, Caselles K, et al. Validation of the QR1 Antibody for the Evaluation of PD-L1 Expression in Non-Small Cell Lung Adenocarcinomas. Appl Immunohistochem Mol Morphol 2020;28:23-9. [Crossref] [PubMed]
- Conde E, Caminoa A, Dominguez C, et al. Aligning digital CD8+ scoring and targeted next-generation sequencing with programmed death ligand 1 expression: a pragmatic approach in early-stage squamous cell lung carcinoma. Histopathology 2018;72:270-84. [Crossref] [PubMed]
- Neuman T, London M, Kania-Almog J, et al. A Harmonization Study for the Use of 22C3 PD-L1 Immunohistochemical Staining on Ventana’s Platform. J Thorac Oncol 2016;11:1863-8. [Crossref] [PubMed]
- Røge R, Vyberg M, Nielsen S. Accurate PD-L1 Protocols for Non–Small Cell Lung Cancer can be Developed for Automated Staining Platforms With Clone 22C3 Appl Immunohistochem. Mol Morphol 2017;25:381-5. [Crossref] [PubMed]
- Savic S, Berezowska S, Eppenberger-Castori S, et al. PD-L1 testing of non-small cell lung cancer using different antibodies and platforms: a Swiss cross-validation study. Virchows Arch 2019;475:67-76. [Crossref] [PubMed]
- Koomen BM, Badrising SK, van den Heuvel MM, et al. Comparability of PD‐L1 immunohistochemistry assays for non‐small cell lung cancer: a systematic review. Histopathology 2020;76:793-802. [Crossref] [PubMed]
- Ilie M, Khambata-Ford S, Copie-Bergman C, et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PloS One 2017;12:e0183023. [Crossref] [PubMed]
- Keller MD, Neppl C, Irmak Y, et al. Adverse prognostic value of PD-L1 expression in primary resected pulmonary squamous cell carcinomas and paired mediastinal lymph node metastases. Mod Pathol 2018;31:101-10. [Crossref] [PubMed]
- Russell-Goldman E, Kravets S, Dahlberg SE, et al. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathol 2018;126:253-63. [Crossref] [PubMed]
- Butter R, ’t Hart NA, Hooijer GKJ, et al. Multicentre study on the consistency of PD-L1 immunohistochemistry as predictive test for immunotherapy in non-small cell lung cancer. J Clin Pathol 2020;73:423-30. [Crossref] [PubMed]
- Clavé S, Pijuan L, Casadevall D, et al. CD274 (PDL1) and JAK2 genomic amplifications in pulmonary squamous-cell and adenocarcinoma patients. Histopathology 2018;72:259-69. [Crossref] [PubMed]
- Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS -Mutant Lung Adenocarcinoma. Cancer Discov 2018;8:822-35. [Crossref] [PubMed]
- Skoulidis F, Byers LA, Diao L, et al. Co-occurring Genomic Alterations Define Major Subsets of KRAS-Mutant Lung Adenocarcinoma with Distinct Biology, Immune Profiles, and Therapeutic Vulnerabilities. Cancer Discov 2015;5:860-77. [Crossref] [PubMed]
- Biton J, Mansuet-Lupo A, Pécuchet N, et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti–PD-1 in Lung Adenocarcinoma. Clin Cancer Res [Internet]. Available online: http://clincancerres.aacrjournals.org/lookup/doi/10.1158/1078-0432.CCR-18-0163
- Koh J, Go H, Keam B, et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol 2015;28:1154-66. [Crossref] [PubMed]
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018;48:434-52. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Nakamura S, Hayashi K, Imaoka Y, et al. Intratumoral heterogeneity of programmed cell death ligand-1 expression is common in lung cancer. PloS One 2017;12:e0186192. [Crossref] [PubMed]
- Thunnissen E, Kerr KM, Dafni U, et al. Programmed death-ligand 1 expression influenced by tissue sample size. Scoring based on tissue microarrays’ and cross-validation with resections, in patients with, stage I-III, non-small cell lung carcinoma of the European Thoracic Oncology Platform Lungscape cohort. Mod Pathol 2020;33:792-801. [Crossref] [PubMed]
- Munari E, Zamboni G, Sighele G, et al. Expression of programmed cell death ligand 1 in non-small cell lung cancer: Comparison between cytologic smears, core biopsies, and whole sections using the SP263 assay. Cancer Cytopathol 2019;127:52-61. [Crossref] [PubMed]
- Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016;27:147-53. [Crossref] [PubMed]
- Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a Promising Method for the Evaluation of Tumor PD-L1 Expression in Lung Cancer. Clin Lung Cancer 2017;18:527-34.e1. [Crossref] [PubMed]
- Hardy LB, Fitzgibbons PL, Goldsmith JD, et al. Immunohistochemistry validation procedures and practices: a College of American Pathologists survey of 727 laboratories. Arch Pathol Lab Med 2013;137:19-25. [Crossref] [PubMed]
- Goldsmith JD, Fitzgibbons PL, Swanson PE. Principles of Analytic Validation of Clinical Immunohistochemistry Assays. Adv Anat Pathol 2015;22:384-7. [Crossref] [PubMed]
- Fitzgibbons PL, Goldsmith JD, Souers RJ, et al. Analytic Validation of Immunohistochemical Assays: A Comparison of Laboratory Practices Before and After Introduction of an Evidence-Based Guideline. Arch Pathol Lab Med 2017;141:1247-54. [Crossref] [PubMed]
- Stuart LN, Volmar KE, Nowak JA, et al. Analytic Validation of Immunohistochemistry Assays: New Benchmark Data From a Survey of 1085 Laboratories. Arch Pathol Lab Med 2017;141:1255-61. [Crossref] [PubMed]
- Torlakovic EE, Cheung CC, D’Arrigo C, et al. Evolution of Quality Assurance for Clinical Immunohistochemistry in the Era of Precision Medicine - Part 2: Immunohistochemistry Test Performance Characteristics. Appl Immunohistochem Mol Morphol 2017;25:79-85. [Crossref] [PubMed]
- Cooper WA, Russell PA, Cherian M, et al. Intra- and Interobserver Reproducibility Assessment of PD-L1 Biomarker in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4569-77. [Crossref] [PubMed]
- Chang S, Park HK, Choi YL, et al. Interobserver Reproducibility of PD-L1 Biomarker in Non-small Cell Lung Cancer: A Multi-Institutional Study by 27 Pathologists. J Pathol Transl Med 2019;53:347-53. [Crossref] [PubMed]


