Overview of clinicopathologic features of ALK-rearranged lung adenocarcinoma and current diagnostic testing for ALK rearrangement
Introduction
The echinoderm microtubule-associated protein-like 4 and the anaplastic lymphoma kinase (EML4-ALK) fusion genes were identified in non-small cell lung cancer (NSCLC) in 2007 (1). The presence of ALK fusion in NSCLC is the best predictor of response to crizotinib, an ALK tyrosine kinase inhibitor (2,3), and these data led to the accelerated approval of crizotinib by the U.S. Food and Drug Administration (FDA). The incidence of the ALK rearrangement in NSCLC has been reported to be approximately 5% in various studies (1,4-6). Several studies showed particular clinical characteristics of patients with ALK-rearranged NSCLC (7-9). In addition, ALK-rearranged tumors were associated with histomorphologic features and positive correlation with histologic subtypes using the new International Association for the Study of Lung Cancer, American Thoracic Society and European Respiratory Society (IASLC/ATS/ERS) lung adenocarcinoma (ADC) classification (4,10-13).
Currently, the three primary methods of detecting ALK rearrangements are fluorescent in situ hybridization (FISH), immunohistochemistry (IHC), and the reverse transcriptase polymerase chain reaction (RT-PCR). Each of these individual methods has both advantages and disadvantages. There are many efforts to improve the sensitivity of identifying ALK rearrangement and recently, the Ventana ALK assay is a new method of detecting ALK rearrangements with high sensitivity (14).
This review is focused on clinicopathologic features of ALK-rearranged lung ADC and current diagnostic testing for ALK rearrangement.
ALK gene rearrangement in NSCLC
The EML4-ALK fusion in NSCLC results from an inversion in the short arm of chromosome two, fusing the N-terminal domain of EML4 to the intracellular kinase domain of ALK (3' gene region), resulting in a constitutively active ALK tyrosine kinase (1). EML4-ALK fusion gene, by itself, is a potent oncogenic driver, reported in about 3-7% of all NSCLC patients. Other fusion partners for ALK have been discovered in NSCLC, such as TFG-ALK (15), KIF5B-ALK (16), and KCL1-ALK (17), and multiple EML4-ALK isoforms (18-20) have been identified, but their clinical significance still remains unknown.
Clinicopathologic characteristics of ALK-rearranged NSCLC
ALK rearrangements are more often found in never or light ex-smokers, in younger age patients and in lung ADC. Published studies have consistently reported that young age and history of never smoking are statistically different between patients with ALK-rearranged and ALK-negative lung ADCs (6,8,21,22). Although approximately 70-80% of ALK-rearranged patients are nonsmokers, the remaining 20-30% includes ex- or current smokers. Some previous studies, however, found that the ALK rearrangements were not associated with non-smoking (23,24). The age range of ALK-rearranged patients is commonly lower than NSCLC patients’ and even younger than the EGFR-mutated population (25). A major challenge is that a younger age at presentation and a lack of smoking history of patients with tumors harboring ALK rearrangement are overlapped characteristic of those who harbor EGFR mutations.
In our previous study, ALK-rearranged tumors exhibited aggressive behavior such as nodal metastasis and advanced disease stage at diagnosis (25,26). In another study, they also observed a strong association of ALK rearrangement with advanced stage in NSCLC patients (27), which strengthened the importance of ALK testing in advanced stage disease.
Distinct histomorphologic features of ALK-rearranged lung ADC
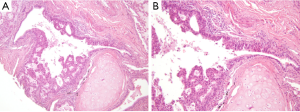
Several studies have investigated the predictive value of pathological and morphological features in detecting ALK-rearranged tumors. Although the results of these studies have been inconsistent because of the limited number of ALK-rearranged tumors, solid signet-ring cell and cribriform pattern has been known to be associated with ALK rearrangement in lung ADC (7,9-12,28). In our previous study, ALK-rearranged lung ADC exhibited several histological characteristics that differentiated it from other genotypes: cribriform formation, presence of mucin-containing cells and presence of psammoma bodies (25). We also identified a close relationship to the adjacent bronchial epithelium is a unique feature of ALK-rearranged tumors. In some ALK-rearranged cases, tumor cells invaded the adjacent bronchiolar epithelium and showed the appearance of “budding off” of small epithelial cell clusters into the lumen. Furthermore, flat atypical lesions that resembled adjacent tumor cells infiltrated the non-neoplastic bronchial epithelium (Figure 1). ALK-rearranged tumors were more likely to be centrally located and easily obtained from the bronchoscopic biopsy procedure. Our findings suggest that ALK-rearranged tumors might be originated from different cell type, in contrast to EGFR-mutated tumors that is originated terminal respiratory unit (TRU) (29-31). In addition, frequent immunoexpression of p63 as well as TTF-1 in ALK-rearranged tumors has been described in a few studies (10,25) (Figure 2). Although the frequent reactivity to TTF-1 in ALK-rearranged tumors indicates derivation from TRU (31), type II pneumocytes or Clara cells native to that unit are typically negative for p63 (32). We proposed that a cell type that dually expresses TTF-1 and p63, as a cell of origin of ALK-rearranged tumors and overexpression of p63 might have functional roles related with carcinogenesis or tumor differentiation in specific subset of lung ADCs, however, a specific cell type of ALK-rearranged tumors has not yet been elucidated.
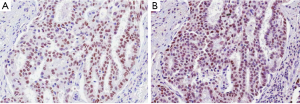
A few studies have reported a positive histological correlation with ALK rearrangement in lung ADC using the new IASLC/ATS/ERS classification that was published in 2011 (4,10-13). The solid subtype was significantly more frequent in the ALK-rearranged cancers, however, an ALK-positive rate is about 8% among the solid subtype ADCs that is similar with 9% in acinar subtype (SNUBH unpublished data). In our study, ALK-rearranged lung ADCs were also significantly associated with solid predominant subtype and not with acinar or papillary predominant subtypes (33). Another study showed that the existence of a minor mucinous component was independently associated with a relatively high prevalence of ALK rearrangement (34). However, no morphological characteristics could identify a specific genetic subtype, suggesting that genetic alterations are associated with a spectrum of morphological features.
Diagnostic methods for detecting ALK gene rearrangement
Currently three main methods of detecting ALK rearrangement are FISH, IHC, and the RT-PCR.
FISH has been considered the gold standard method for detecting ALK rearrangement. The FDA in the USA approved the Abbot Vysis ALK Break Apart FISH Probe Kit for companion diagnostic testing for ALK-rearranged NSCLC. Although FISH can detect rearrangements regardless of the fusion partners, it is expensive, generally requires specialized technical resources and expertise and thus cannot be applied in all pathological laboratories. In clinical practice, it is important to determine the presence of an ALK rearrangement in small biopsy samples with advanced stage NSCLC patients. Therefore, FISH analysis may not be available for screening all NSCLC patients.
Alternatively, IHC is less expensive and less time-consuming than ALK FISH, and is a well-established method in the routine work of every pathology department. IHC is less sensitive than FISH analysis to variations in handling or pretreatment of specimens, and a diagnosis can be established with a smaller number of tumor cells than required for FISH analysis. Several antibodies and detection systems have been investigated for overcoming the low expression level of the ALK fusion protein (35-37). 5A4 and D5F3 are known to be high affinity antibody clones (Figure 3) (35-38). Recently, the novel fully automated ALK IHC assay developed by Ventana company has been introduced that uses D5F3 antibody and relies on the tyramide amplification technique bound to the Ventana automated BenchMark XT for high sensitivity (Figure 3B). Several studies have demonstrated that there is a high concordance between the Ventana IHC and FISH (14,39,40). In September 2013, this automated IHC of Ventana Company has received China’s FDA approval as a companion diagnostic identifying ALK protein expression in lung cancer patients.
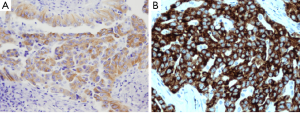
The RT-PCR is a more sensitive and rapid method that can identify specific variants of the ALK rearrangements. However, RT-PCR requires ALK fusion variants to be known so that primers to all variants are included in the reaction. Although with an ever-expanding list of ALK fusion variants, all the reported variants require skillful application. In addition, majority of current ALK fusion variants were detected by RT-PCR in fresh frozen tumor tissue. However, in daily clinical practice, most of the tumor tissue available for molecular profiling is from FFPE tissue, where the integrity of RNAs is likely to be greatly compromised compared with fresh frozen tissue. Although FISH and IHC can be performed on a single FFPE slide, RT-PCR requires multiple slides in order to extract sufficient RNA for a successful reaction. Therefore, detecting ALK rearrangements using RT-PCR continues to be challenging in routine practice.
ALK testing in routine practice
Currently, crizotinib therapy is indicated only in ALK-rearranged NSCLC patients who are either inoperable or have residual or recurrent disease after surgery. However, the majority of lung cancer patients present with advanced stage and at the time of initial biopsy many patients have not been fully-staged or assessed for surgery. In this context, the guidelines for molecular testing in lung cancer recently published by the College of American Pathologists (CAP), IASLC, and Association for Molecular Pathology (AMP) has recommended performing ALK FISH testing at the time of diagnosis for patients presenting with advanced-stage NSCLC who are suitable for medical therapy or at a time of recurrence (41). That is, reflex ALK testing in all lung cancer patients would be encouraged but is only possible if it can be performed in a cost effective and timely manner.
As a companion diagnostic test, reflex ALK FISH in all lung cancer patients would be desirable, however, this strategy in the routine practice is difficult due to several limitations such as cost, resource and time constraints. Recently, many retrospective studies have suggested that ALK IHC can be used as a screening test for ALK gene rearrangements in lung cancer (6,35,38,42-45). Thus, reflex ALK IHC followed by confirmatory FISH testing can be readily integrated into the routine clinical setting and represents a cost effective and practical approach to screening for this druggable gene rearrangement. For the successful reflex test of ALK, we caution that ALK IHC should be fully validated in individual laboratories, performed with appropriate lung specific protocols when applied in clinical setting and controlled based on the results of the test. Even if IHC result is negative, FISH studies can still be performed on patients with a high clinical suspicion of ALK gene rearrangement.
ALK testing of rebiopsy samples during disease progression in patients treated by ALK inhibitor
Many of advanced NSCLC harboring ALK gene rearrangement treated with ALK inhibitors eventually relapse due to acquired resistance. Identifying the various mechanisms of resistance is critical to developing new treatment strategies in the acquired resistance setting. Several studies have identified several resistance mechanisms to crizotinib in rearranged EML4-ALK NSCLC and more studies are needed to fully understand the resistance mechanisms and to define new targeted strategies (46-48). This resistance has been associated with various tumoral genetic changes, such as other mutations in the ALK gene, ALK gene amplification or activating mutations of other genes (49). These changes may guide the selection of further treatments in these patients with resistant tumors. Therefore, it is widely accepted that rebiopsy is useful at the time of progression. However, this depends on the feasibility of rebiopsy at this time. Bosc et al. evaluated the percentage of patients who underwent rebiopsy with mutant EGFR or ALK-rearranged NSCLC and acquired resistance to tyrosine kinase inhibitors (50). A rebiopsy was considered as feasible in 72% while a biopsy was in fact performed in 46%. When rebiopsy was performed, there was sufficient tumor material in the vast majority of cases (more than 85%) in several studies (50,51).
There were few contraindications to biopsy, reflecting the fact that patients with activating mutations are often nonsmokers or former light smokers and therefore less prone to tobacco related comorbid conditions such as COPD and heart disease. The most frequent constraint was poor physical condition, probably associated with cancer progression.
It should be considered that some degree of heterogeneity may occur between the primary tumor and its metastases. We previously found ALK protein expression in 11.9% (8/67) of primary NSCLCs and 25.4% (17/67) of their matched metastatic lesions, indicating that metastatic progression can be associated changes in ALK expression (52). Regarding the biopsy site, some authors consider that the highest failure rates are observed when the tissue is obtained from bone samples (53). These high failure rates are mostly observed when a decalcification process is needed. Despite significant improvements using EDTA (54), we have to recognize that bone biopsies are still not recommended for molecular testing. Patients and physicians may be reluctant to accept a surgical brain biopsy, even a minimally invasive stereotactic biopsy.
Understanding the molecular mechanisms of resistance and personalizing the treatment accordingly justify the need for rebiopsy. Although a vast majority of patients may undergo a second biopsy procedure, in one third of cases a biopsy was either not feasible, contraindicated or not suitable for molecular analysis. This emphasizes the need for the development of less invasive techniques.
Clinical impact and conclusions
The codevelopment of drug with a companion diagnostic assay has accelerated rapid development in the area of diagnostic assays in lung cancer. This led to the most sensitive, specific, and cost-effective assay for the screening of ALK rearrangement. As well as diagnostic testing, understanding distinct clinical and histomorphological characteristics of ALK-rearranged lung cancer may improve diagnostic accuracy and help us to detect all patients with ALK-rearranged lung cancer.
With the advances in acquired resistance after crizotinib therapy, the importance of repeat tissue acquisition and molecular testing during disease progression and the need for close collaboration between pathologists and clinicians are increasing.
Acknowledgements
Funding: This work was supported by a Grant-in-Aid from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2013-059757), and the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C1907).
Disclosure: The authors declare no conflict of interest.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [PubMed]
- Bang YJ. Treatment of ALK-positive non-small cell lung cancer. Arch Pathol Lab Med 2012;136:1201-4. [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13-7. [PubMed]
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. [PubMed]
- Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011;6:466-72. [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23. [PubMed]
- Yoshida A, Tsuta K, Watanabe S, et al. Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer 2011;72:309-15. [PubMed]
- Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011;35:1226-34. [PubMed]
- Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol 2012;25:1462-72. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Wynes MW, Sholl LM, Dietel M, et al. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol 2014;9:631-8. [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [PubMed]
- Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 2009;15:3143-9. [PubMed]
- Togashi Y, Soda M, Sakata S, et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PLoS One 2012;7:e31323. [PubMed]
- Choi YL, Takeuchi K, Soda M, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res 2008;68:4971-6. [PubMed]
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [PubMed]
- Sanders HR, Li HR, Bruey JM, et al. Exon scanning by reverse transcriptase-polymerase chain reaction for detection of known and novel EML4-ALK fusion variants in non-small cell lung cancer. Cancer Genet 2011;204:45-52. [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [PubMed]
- Wang Z, Zhang X, Bai H, et al. EML4-ALK rearrangement and its clinical significance in Chinese patients with advanced non-small cell lung cancer. Oncology 2012;83:248-56. [PubMed]
- Li Y, Li Y, Yang T, et al. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PLoS One 2013;8:e52093. [PubMed]
- Koh Y, Kim DW, Kim TM, et al. Clinicopathologic characteristics and outcomes of patients with anaplastic lymphoma kinase-positive advanced pulmonary adenocarcinoma: suggestion for an effective screening strategy for these tumors. J Thorac Oncol 2011;6:905-12. [PubMed]
- Kim H, Jang SJ, Chung DH, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 2013;8:e76999. [PubMed]
- Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. [PubMed]
- Kim TJ, Park CK, Yeo CD, et al. Simultaneous diagnostic platform of genotyping EGFR, KRAS, and ALK in 510 Korean patients with non-small-cell lung cancer highlights significantly higher ALK rearrangement rate in advanced stage. J Surg Oncol 2014;110:245-51. [PubMed]
- Popat S, Gonzalez D, Min T, et al. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer 2012;75:300-5. [PubMed]
- Yatabe Y, Mitsudomi T. Epidermal growth factor receptor mutations in lung cancers. Pathol Int 2007;57:233-44. [PubMed]
- Yatabe Y, Kosaka T, Takahashi T, et al. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol 2005;29:633-9. [PubMed]
- Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol 2002;26:767-73. [PubMed]
- Wu M, Orta L, Gil J, et al. Immunohistochemical detection of XIAP and p63 in adenomatous hyperplasia, atypical adenomatous hyperplasia, bronchioloalveolar carcinoma and well-differentiated adenocarcinoma. Mod Pathol 2008;21:553-8. [PubMed]
- Kim H, Park E, Kim YJ, et al. ALK rearrangement in a pure squamous cell carcinoma: the challenge of detection of ALK rearrangement. Virchows Arch 2013;462:597-9. [PubMed]
- Hu H, Pan Y, Li Y, et al. Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. Onco Targets Ther 2014;7:1423-37. [PubMed]
- Conklin CM, Craddock KJ, Have C, et al. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol 2013;8:45-51. [PubMed]
- Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 2010;16:1561-71. [PubMed]
- Selinger CI, Rogers TM, Russell PA, et al. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol 2013;26:1545-53. [PubMed]
- Minca EC, Portier BP, Wang Z, et al. ALK status testing in non-small cell lung carcinoma: correlation between ultrasensitive IHC and FISH. J Mol Diagn 2013;15:341-6. [PubMed]
- Ying J, Guo L, Qiu T, et al. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Ann Oncol 2013;24:2589-93. [PubMed]
- Alì G, Proietti A, Pelliccioni S, et al. ALK Rearrangement in a Large Series of Consecutive Non-Small Cell Lung Cancers: Comparison Between a New Immunohistochemical Approach and Fluorescence In Situ Hybridization for the Screening of Patients Eligible for Crizotinib Treatment. Arch Pathol Lab Med 2014;138:1449-58. [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. [PubMed]
- Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459-65. [PubMed]
- Han XH, Zhang NN, Ma L, et al. Immunohistochemistry reliably detects ALK rearrangements in patients with advanced non-small-cell lung cancer. Virchows Arch 2013;463:583-91. [PubMed]
- Martinez P, Hernández-Losa J, Montero MÁ, et al. Fluorescence in situ hybridization and immunohistochemistry as diagnostic methods for ALK positive non-small cell lung cancer patients. PLoS One 2013;8:e52261. [PubMed]
- McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol 2012;7:348-54. [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [PubMed]
- Kim S, Kim TM, Kim DW, et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol 2013;8:415-22. [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Bosc C, Ferretti GR, Cadranel J, et al. Rebiopsy during disease progression in patients treated by TKI for oncogene-addicted NSCLC. Target Oncol 2014. [Epub ahead of print].
- Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 2011;17:1169-80. [PubMed]
- Kim H, Xu X, Yoo SB, et al. Discordance between anaplastic lymphoma kinase status in primary non-small-cell lung cancers and their corresponding metastases. Histopathology 2013;62:305-14. [PubMed]
- Vanderlaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer 2014;84:39-44. [PubMed]
- Singh VM, Salunga RC, Huang VJ, et al. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann Diagn Pathol 2013;17:322-6. [PubMed]


