Immunotherapy as a treatment for small cell lung cancer: a case report and brief review
Introduction
Small cell lung cancer (SCLC) accounts for about 15% of all lung cancer cases (1). It is an aggressive disease, characterized by the extensive spread of early metastasis. According to the International Association for Lung Cancer Research (IASLC) standard ‘TNM’ staging, SCLC can be classified into 2 stages: limited stage (LS-SCLC) and extended stage (ES-SCLC). Approximately 70% of SCLC patients are classified as having ES-SCLC (2,3).
The standard initial treatment for advanced ES-SCLC is platinum-based chemotherapy, which has a high response rate (RR) but a transient response overall (4,5). Patients whose tumors recur more than 6 months after first-line chemotherapy can benefit from repeated treatment using the same original regimen (6). Topotecan, with a RR of 15–20% and a 1-year overall survival (OS) rate of 30%, is the second-line treatment for SCLC (7). The options for subsequent lines of treatment are limited, meaning there is an urgent demand for new treatments for patients with SCLC. The majority of SCLC patients are characterized by smoking exposure, high tumor mutational burden (TMB), and high neoantigens formation, all of which may impact the effectiveness of immunotherapy.
At present, studies have been conducted on the two immune checkpoint inhibitor (ICI) pathways—programmed death receptor-1/ligand 1 (PD-1/L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4)—as well as combined therapy for the two pathways. In the studies conducted to date, immunotherapy has been shown to have positive effects as part of the treatment of SCLC patients. Results of IMPOWER-133, for example, showed a longer progression-free survival (PFS) and OS in patients receiving etoposide/carboplatin/atezolizumab (8). Subsequently, many clinical studies in which immunotherapy is combined with chemotherapy as the first-line treatment of SCLC, such as the CASPIAN study, are ongoing. In our case report, we observed a patient treated with chemotherapy combined with immunotherapy who achieved partial response (PR), and then subsequently conducted a review of studies which have focused on the role of ICIs in SCLC.
Case presentation
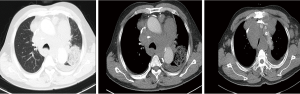
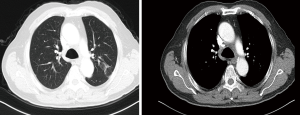
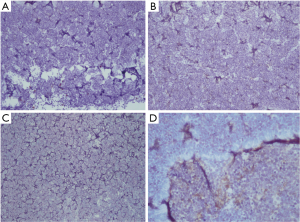
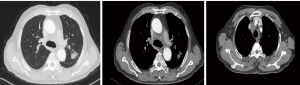
On September 27, 2018, a 68-year-old man was admitted to Shanghai Pulmonary Hospital for 1 week, suffering from a cough, chest tightness, and asthma. He had smoked one pack per day for 40 years. A physical examination discovered that one of the patient’s right neck lymph nodes was enlarged. A CT scan of the patient’s chest revealed a mass in the left lung, enlarged mediastinal and left hilum lymph nodes, and left pleural effusion (Figure 1). The biopsy pathology of the patient’s right neck lymph node showed metastatic neuroendocrine carcinoma, SCLC. Immunohistochemistry studies showed expression of CD56, CgA positive (weak) staining, and synaptophysin, CK negative staining. We also stained for PD-1, PD-L1, and CD8+ (Figure 2). Magnetic resonance imaging (MRI) of the patient’s brain revealed no evidence of metastatic disease. The patient was diagnosed with left lung SCLC T2N2M1-Stage IV (right neck lymph node, left pleural effusion) (IASLC 8th Edition) and received treatment with PD-L1 inhibitor plus etoposide plus carboplatin (PD-L1 inhibitor: every 3 weeks on day 1; carboplatin: AUC 5 on day 1; etoposide: 100 mg/m2 on days 1–3). The patient received four cycles of platinum-based doublet chemotherapy combined with PD-L1 inhibitor. Following two cycles of chemotherapy and PD-L1 inhibitor treatment, the patient’s tumor shrank significantly. After the 4 cycles of therapy, the patient was given PD-L1 inhibitor every 3-week as maintenance. PR was achieved, the mediastinal and neck tumor significantly shrank, pleural effusion disappeared, and only one lesion remained in the left lower lobe (Figure 3). The patient’s symptoms of a cough, chest tightness, and asthma disappeared, and his quality of life improved significantly, with moderate adverse events. A CT scan of the patient’s chest indicated that the pulmonary lesions were stable (Figure 4).
A CT scan of the patient’s chest on September 28, 2018 showed a mass in the left lung, with enlarged mediastinal and left hilum lymph nodes, and left pleural effusion.
A CT scan of the patient’s chest on November 12, 2018 showed that the tumor and the mediastinal node lymph nodes had shrunk significantly and pleural effusion had disappeared.
The chest CT scan on November 12, 2018 showed that the tumor in the left lower lobe was stable.
Review of literature
The biological characteristics of SCLC and perspectives of immunotherapy in SCLC
TMB is considered to be a predictive biomarker for ICI therapy in cancer treatment (9). Due to its genomic instability and high mutagenicity, SCLC is extremely heterogeneous. In previous studies on SCLC, a series of high-frequency inactivation mutations of tumor suppressor genes were found; these included TP53 (75–90%) (10), RB1 (60–90%) (11,12), PTEN (2–4%) (13), and activation mutations of the oncogenes PIK3CA, EGFR, and KRAS (14-16). In addition, there was the MYC gene family, EGFR/BCL2 gene amplification and RASSFIA/PTEN/Fhit gene deficiency (17). The majority of SCLC patients mostly have been exposed to tobacco, the carcinogenic effect of which is associated with a higher mutational burden. In whole genome sequencing of NCI-H209, an SCLC cell line, 22 somatic cells acquired replacements, 65 inserts, and deletions, 58 structural variations were detected. In the mutation of the genomic encoding region, as well as in the MLL2 gene, RB1 C706 mutations and destructive mutations of TP53 gene shear position were found (18).
It is likely that SCLC patients have the immunosuppressive phenotype, despite the high rate of somatic tumor mutation. In previous studies, major histocompatibility antigen class I (MHC-I), which potentially rendered patients more sensitive to immune response, was found to be down-regulated in SCLC (19). A decrease or loss of MHC-I expression results in a decrease in antigen presentation to cytotoxic T cells (CTLs), which allows SCLC cells to evade tumor immune response and develop resistance to ICIs. Furthermore, in SCLC, major histocompatibility antigen class II (MHC-II) and immature myeloid cells are not expressed, making patients more likely to have no immune response (20). In summary, low expression of MHC antigens is a negative predictor of immunotherapy response.
The anti-PD-1/PD-L1 pathway
Part of the CD28 immunoglobulin superfamily, PD-1 is a negative co-stimulating molecule which is usually expressed by CD4/CD8 T cells, dendritic cells, B cells, monocytes, and natural killer T cells. PD-L1 (B7-H1), the most important ligand of PD-1. It is a member of the B7 superfamily, which is expressed on the surface of immune cells and epithelial cells. Combined application of PD-1 and PD-L1 can inhibit the activation and proliferation of T cells, trigger T cell apoptosis, induce and maintain immune tolerance and escape of tumor immune (21). Therefore, blocking the PD-1/PD-L1 signal can promote immune response (22).
Nivolumab
By preventing PD-1 from interacting with PD-L1/PD-L2, nivolumab induces an antitumor immune response. In 2014, the food and drug administration (FDA) approved the use of nivolumab for the treatment of advanced melanoma patients. It had been previously approved for the treatment of metastatic NSCLC. Two phase III trials showed that nivolumab held greater benefits in terms of survival than docetaxel. In 2015, the FDA also approved nivolumab for treating advanced or metastatic squamous cells (23,24).
Treatment for the PD-1/PD-L1 axis was first reported in a multi-cohort study which included SCLC. In the study Checkmate-032, 216 SCLC patients with unknown PD-L1 status were divided into three different cohorts. In the single-agent cohort, the objective RR was 11.9% and 17.2% of patients showed no progress at 6 months. OS at 12 and 18 months was 28.3% and 20.0%, respectively. The incidence of grade 3–4 treatment-related adverse events was 11.9%. Three patients (2.8%) discontinued in the study due to treatment-related adverse events. Nivolumab was found to have long-lasting efficacy and good tolerance in the treatment of recurrent SCLC (25).
Checkmate 451, a phase III randomized double-blind study, enrolled approximately 810 patients with ES-SCLC. The primary endpoints included OS and PFS, and the results have not yet been published (26).
Checkmate 331, an ongoing study, aims to compare nivolumab monotherapy versus chemotherapy as a second-line therapy for 480 patients with relapsed SCLC whose disease progressed after prior advance treatment in the phase III open-label randomized trial. The primary endpoint is OS and the results have not been published yet (27).
Pembrolizumab
The FDA approved pembrolizumab to treat metastatic or inoperable melanoma in 2014. Recently, pembrolizumab has also been used to benefit lung cancer patients who got progressed disease after first-line chemotherapy and those with ALK rearrangement or EGFR positive mutation lung cancer who were progressed after targeted therapy.
Keynote-604 enrolled 453 patients with ES-SCLC in phase III randomized, double-blind placebo-controlled trial to detected first-line pembrolizumab with 4 cycles of chemotherapy until PD was reported. The primary endpoints were PFS and OS. The study is ongoing and will be published in 2020 (28).
Many other first-line SCLC trials have also studied the effects of pembrolizumab. Phase I Keynote-011 used pembrolizumab in combination with standard chemotherapy to treat SCLC. The results will be published in 2020. Phase II clinical trial REACTION was an open, multi-center, randomized controlled trial which compared the efficacy of chemotherapy in combination with pembrolizumab or placebo. PFS was the primary endpoint. Preliminary results will be published in 2020.
NCI-2015-00107 was a phase II trial for single-arm maintenance of pembrolizumab in ES-SCLC. The primary endpoint was PFS. The median PFS was 1.4 months, and the 1-year PFS was 13%. The expression of PD-L1 in tumor stroma was detected in 20/30 cases. The median PFS in patients with positive PD-L1 expression in tumor interstitial interface was 6.5 months. In contrast, the median PFS in patients with negative PD-L1 expression in tumor interstitial interface was 1.3 months. Overall, maintaining pembrolizumab did not improve PFS. However, the 1-year PFS and OS rates were 13% and 37%, respectively. This suggests that some patients could benefit from treatment with pembrolizumab (29).
Keynote-028 studied the effects of pembrolizumab on SCLC. This study only recruited PD-L1 positive (>1%) patients. Twenty-four ES-SCLC patients with at least one previous platinum treatment were treated with pembrolizumab. The overall response rate (ORR) was 33%. In this cohort, the median reaction duration was 19.4 months. The median OS was 9.7 months and the 12-month OS rate was close to 40%. Pembrolizumab is awaiting larger trials for its use as a single-agent treatment in second-line therapy.
Keynote-158 enrolled 107 ES-SCLC patients who received pembrolizumab as a second-line therapy. The primary endpoints were ORR. DOR and PFS. Forty-two (39%) cases were PD-L1 positive and 50 (47%) cases were PD-L1 negative. ORR was 18.7%. In general, the median DOR was not reached. Twelve patients (77%) had DOR ≥9 months. The median PFS of all patients was 2.0 months. The median PFS of PD-L1 positive and PD-L1 negative patients was 2.1 and 1.9 months, respectively. In this study, pembrolizumab exhibited significant antitumor activity and had a sustained response in SCLC, particularly amongst tumor PD-L1 positive expression patients (30).
Atezolizumab
Atezolizumab is a monoclonal antibody against IgG1 isotype of PD-L1. Atezo-monotherapy has shown good safety and efficacy in many tumor types. Preclinical and phase I data suggests that atezolizumab may have synergistic effects, leading to long-lasting responses in NSCLC when used alongside platinum-based chemotherapy.
IMPOWER 133 included 403 patients with ES-SCLC. The study was a phase I/III trial which evaluated the efficacy and safety of the first-line atezolizumab when used in combination with chemotherapy. The primary endpoints were PFS and OS. The median OS was 12.3 months in the atezolizumab arm versus 10.3 months in the placebo arm. The median PFS was 5.2 months in the atezolizumab group versus 4.3 months in the placebo group. Studies showed that in the first-line treatment of ES-SCLC, atezolizumab used in combination with chemotherapy could significantly improve survival (8,31).
Fct-1603 was a phase II study compared Atezolizumab with chemotherapy. In this study, 73 SCLC patients were randomized to Atezolizumab and chemotherapy. The primary endpoint was ORR at 6 weeks, the secondary endpoint was PFS. PFS was 1.4 months with Atezolizumab and 4.2 months with chemotherapy, respectively. The screening biomarker was PD-L1 (32). There was no efficacy or safety of Atezolizumab in recurrent SCLC. Follow-up data will be updated.
Durvalumab
Durvalumab is cancer immunotherapy developed by MedImmune/AstraZeneca, which blocks PD-L1 with PD-1 and CD80 (B7.1). It demonstrated a certain degree of safety and clinical activity when used before the initial treatment of SCLC patients and was studied in CASPIAN, a phase III, randomized trial.
The anti-CTLA-4 pathway
CTLA-4 inhibitors currently include tremelimumab and ipilimumab, the latter being the earliest agent to be used as a clinical ICI, as approved by the FDA.
Ipilimumab
Ipilimumab can effectively block the binding of CTLA-4 to its ligand. It was reported that the irPFS and PFS of patients with lung cancer treated by phased ipilimumab combined with paclitaxel and carboplatin showed improvements. This supports the call for further research into ipilimumab in NSCLC.
Ca184-156 was a phase III randomized double-blind study to evaluate the safety and efficacy of ipilimumab or placebo when used in combination with chemotherapy to treat newly diagnosed ES-SCLC. In this study, 1,132 patients were recruited and randomized (33).
Tremelimumab
CASPIAN was a phase III randomized, multicenter, open-label study, which was designed to assess the efficacy of Durvalumab or Tremelimumab and Durvalumab in combination with chemotherapy. The main endpoints included PFS and OS, and the results are pending (34).
Conclusions
For decades, there has been no substantial progress in the treatment of SCLC, and previous trials have shown that immunotherapy with checkpoint inhibitors may be an effective way to control disease over the long term. Although combination therapy is more effective, therapeutic toxicity must also be considered, particularly in SCLC patients outside of clinical trials, who are typically in a poor ECOG state. Therefore, determining which patients could benefit from immunotherapy remains an important research objective. Although early results have suggested that high TMB and positive expression of PD-L1 may be conducive to the selection of CTLA-4/PD-1 inhibitor combination, no consensus has been reached and more relevant evidence is required. The results of the large phase III trial currently underway are eagerly awaited.
Acknowledgments
Funding: This study was supported in part by a grant from the National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from the Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), the National Key Research & Development Project (2016YFC0902300), Major disease clinical skills enhancement program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor” Outstanding Young Talents Program (fkyq1901), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), grant of Shanghai Science and Technology Commission (16JC1405900).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.20). CZ serves as an unpaid Editor-in-Chief of Translational Lung Cancer Research. YH serves as the unpaid editorial board member of Translational Lung Cancer Research from Jan 2020 to Dec 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang W, Girard L, Zhang YA, et al. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl Lung Cancer Res 2018;7:32-49. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012;13:115-22. [Crossref] [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282-91. [Crossref] [PubMed]
- Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol 1987;23:1697-9. [Crossref] [PubMed]
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086-92. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol 2001;28:3-13. [Crossref] [PubMed]
- Mori N, Yokota J, Akiyama T, et al. Variable mutations of the RB gene in small-cell lung carcinoma. Oncogene 1990;5:1713-7. [PubMed]
- Arriola E, Cañadas I, Arumí M, et al. Genetic changes in small cell lung carcinoma. Clin Transl Oncol 2008;10:189-97. [Crossref] [PubMed]
- Kim SK, Su LK, Oh Y, et al. Alterations of PTEN/MMAC1, a candidate tumor suppressor gene, and its homolog, PTH2, in small cell lung cancer cell lines. Oncogene 1998;16:89-93. [Crossref] [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [Crossref] [PubMed]
- Shibata T, Kokubu A, Tsuta K, et al. Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett 2009;283:203-11. [Crossref] [PubMed]
- Onuki N, Wistuba II, Travis WD, et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999;85:600-7. [Crossref] [PubMed]
- Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015;4:533-44. [PubMed]
- Pleasance ED, Stephens PJ, O'Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184-90. [Crossref] [PubMed]
- Graus F, Dalmou J, Reñé R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol 1997;15:2866-72. [Crossref] [PubMed]
- Antonia SJ, Mirza N, Fricke I, et al. Combination of a p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 2006;12:878-87. [Crossref] [PubMed]
- Travert C, Tomasini P, Jeanson A, et al. First-line pembrolizumab in programmed death ligand 1 positive non-small cell lung cancer. Transl Cancer Res 2019;8:2514-6. [Crossref]
- Chae YK, Arya A, Iams W, et al. Immune checkpoint pathways in non-small cell lung cancer. Ann Transl Med 2018;6:88. [Crossref] [PubMed]
- Horn L, Reck M, Spigel DR. The Future of Immunotherapy in the Treatment of Small Cell Lung Cancer. Oncologist 2016;21:910-21. [Crossref] [PubMed]
- Reck M, Heigener D, Reinmuth N. Immunotherapy for small-cell lung cancer: emerging evidence. Future Oncol 2016;12:931-43. [Crossref] [PubMed]
- Ready N, Farago AF, de Braud F, et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J Thorac Oncol 2019;14:237-44. [Crossref] [PubMed]
- Ready N, Owonikoko TK, Postmus PE, et al. CheckMate 451: A randomized, double-blind, phase III trial of nivolumab (nivo), nivo plus ipilimumab (ipi), or placebo as maintenance therapy in patients (pts) with extensive-stage disease small cell lung cancer (ED-SCLC) after first-line platinum-based doublet chemotherapy (PT-DC). J Clin Oncol 2016;34:abstr TPS8579.
- Horn L, Reck M, Gettinger SN, et al. CheckMate 331: An open-label, randomized phase III trial of Nivolumab versus chemotherapy in patients (pts) with relapsed small cell lung cancer (SCLC) after first-line platinum-based chemotherapy (PT-DC). J Clin Oncol 2016;34:abstr TPS8578.
- Thomas M, Ponce-Aix S, Navarro Mendivil A, et al. 1527O - Top-line data from the randomized phase 2 IMPULSE study in small-cell lung cancer (SCLC): Immunotherapeutic maintenance treatment with lefitolimod. Ann Oncol 2017;28:v539-42. [Crossref]
- Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II Study of Maintenance Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer (SCLC). J Thorac Oncol 2018;13:1393-9. [Crossref] [PubMed]
- Chung HC, Lopez-Martin JA, Kao SC, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol 2018;36:abstr 8506.
- Horn L, Reck M, Mok TSK, et al. A Phase III study of Atezolizumab with carboplatin plus etoposide in patients with extensive-stage small cell lung cancer (IMpower133). Ann Oncol 2016;27. [Crossref]
- Pujol JL, Greillier L, Audigier Valette C, et al. 1664O A randomized non-comparative phase II study of anti–PD-L1 ATEZOLIZUMAB or chemotherapy as second-line therapy in patients with small cell lung cancer: Results from the IFCT-1603 trial. Ann Oncol 2018;29. [Crossref]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:3740-8. [Crossref] [PubMed]
- Paz-Ares L, Jiang H, Huang Y, et al. CASPIAN: Phase 3 Study of First-Line Durvalumab ± Tremelimumab + Platinum-Based Chemotherapy vs Chemotherapy Alone in ED-SCLC. J Thorac Oncol 2017;12:S2398. [Crossref]