
Non-alcoholic fatty liver disease is associated with immune checkpoint inhibitor-based treatment response in patients with non-small cell lung cancer with liver metastases
Introduction
Immune checkpoint inhibitor (ICI) which target programmed cell death-1 (PD-1) and its ligand (PD-L1) has shown marked anti-tumor activity in a broad range of cancers. The results of the CA209-003 study showed that nivolumab prolonged the overall survival (OS) of patients with pretreated advanced non-small cell lung cancer (NSCLC), with 5-year OS rate of 16% (1). Based on data from the KeyNote-001 study, the 5-year OS rate was as high as 29.6% for patients with a PD-L1 tumor proportion score of ≥50% (2). However, the majority of patients fail to respond to this treatment modality, and as a result, the identification of predictive biomarkers for ICI will be a major focus of research for some time.
Obesity, as a new emerging biomarker, is attracting an increasing amount of attention. Obese patients with melanoma could derive greater survival benefit from immunotherapy (3). A recent study also demonstrated that NSCLC patients with high-body mass index (BMI) had better clinical outcomes than those with low BMI (1-year OS rate: 46.4% vs. 39%) (4). Non-alcoholic fatty liver disease (NAFLD) is an obesity-related disease characterized by the accumulation of local adipose tissue in the liver (5). However, the relationship between NAFLD and ICI still needs to be illuminated. Therefore, we explored the impact of NAFLD on the efficacy of ICI-based treatment.
NAFLD is one of the most common chronic liver diseases, with a prevalence of 13.48–31.79% (5). Generally, NAFLD, is diagnosed by evidence of hepatic steatosis (detected by imaging or histology) in the absence of secondary causes of steatosis or other liver diseases, such as excessive alcohol consumption, hepatitis, Wilson disease, and hepatotoxic medication (5). NAFLD has been shown to cause inflammatory infiltration, with multiple T-cell subsets involved in the pathogenesis of NAFLD (6). Gadd et al. demonstrated that broad leukocyte subsets contributed to portal inflammation (7). Inzaugarat et al. found that patients with non-alcoholic steatohepatitis had a higher frequency of IFN-γ-producing CD4+ and CD8+ T cells in their peripheral blood (8). These results indicate that our speculation that NAFLD may exert effect on the treatment of ICI is reasonable.
NAFLD is proved to disrupt the liver regional immune microenvironment, which could affect the progression of cancer (9). Luo et al. reported that the levels of STING were increased in liver macrophages from patients with NAFLD (10). Moreover, Wu et al. suggested hepatic steatosis to be an independent predictor of liver metastasis in NSCLC patients (11). Therefore, we hypothesized that NAFLD affects the development of liver metastases (LMs).
LMs have always been a subject of concern, mainly due to patients with LMs usually having a poorer prognosis compared to patients with metastases at other sites (12,13). The therapeutic benefit of ICI-based treatment is also limited in patients with LMs (14-15). Tumeh et al. reported that LMs status was associated with reduced responses and shortened PFS in NSCLC patients treated with NSCLC (16). Thus, identifying potential beneficiaries of ICI-based therapy from within this population is of clinical importance.
In the present study, we aimed to compare the clinical outcomes of NSCLC patients with and without NAFLD who underwent ICI-based treatment, with LMs as a critical stratified factor.
Methods
Study population
We retrospectively reviewed the medical records of NSCLC patients treated at Shanghai Pulmonary Hospital between June 2015 and June 2019. NAFLD was confirmed by the ultrasound examination of abdomen. The criteria for inclusion were as follows: (I) confirmed NSCLC by pathology; (II) stage IIIB/IV according to the eighth edition of the TNM Classification for lung cancer; (III) with measurable lesions; and (IV) had received ICI-based treatment. Patients to whom any of the following criteria applied were excluded from the study: (I) EGFR/ALK/ROS1 alterations; (II) hepatitis virus infection; (III) a history of heavy drinking (>14 drinks per week for women and >21 drinks per week for men); (IV) had received other immunotherapy, including vaccines and adoptive cellular immunotherapy; (V) had other malignant tumors; (VI) lack of liver imaging evaluation; or (VII) lost to follow-up before evaluation of treatment efficacy.
Because of the study’s retrospective nature, the need for written informed consent was waived. The study was approved by the institutional review board of Shanghai Pulmonary Hospital.
Definitions of variables
The efficacy of ICI-based treatment was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Disease control rate (DCR) was defined as complete plus partial response plus stable disease, and objective response rate (ORR) as complete response plus partial response. Progression-free survival (PFS) was defined as the interval from the initiation of ICI treatment to confirmed disease progression or death from any cause. If disease progression had not occurred before the deadline for analysis or last follow-up date, then the data were censored.
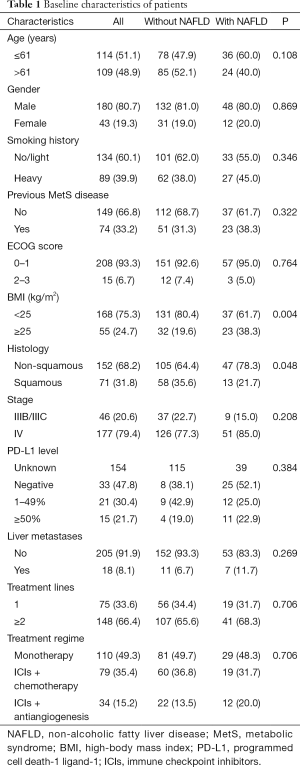
The expression level of PD-L1 was detected with 22C3-antibody. Because of the strong bidirectional association between NAFLD and metabolic syndrome (MetS) diseases, such as diabetes, hypertension, and hyperlipemia (17), if a patient had any one of these diseases, then previous MetS disease was recorded as “yes” (Table 1). BMI was also collected, with 25 kg/m2 serving as the cut-off value, based on the Chinese patients in our study having a much lower BMI than Western patients.
Full table
Statistical analysis
Statistical analyses were carried out using SPSS version 22.0. For continuous variables, the Mann-Whitney U test was applied to make comparisons between groups, and Pearson’s χ2 or Fisher’s exact tests were used for categorical variables. In the univariate analysis, the Kaplan-Meier method was employed to estimate PFS and OS and the log-rank test was used to make comparisons. In the multivariate analysis, the Cox proportional hazards model was used to calculate the hazard ratio (HR) and corresponding 95% confidence interval (CI). Statistical significance was indicated by a two-sided P value <0.05.
Results
Patient characteristics
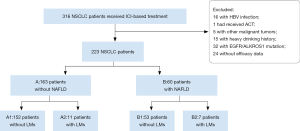
A total of 223 patients with advanced NSCLC who received ICI-based treatment at Shanghai Pulmonary Hospital were involved in the study. Of the patients, 26.9% (n=60) were confirmed to have NAFLD by ultrasound examination of abdomen at the initiation ICI-based treatment (Figure 1). The baseline characteristics of the patients with and without NAFLD, including age, gender, smoking history, previous MetS disease, ECOG score, stage, PD-L1 level, LMs, treatment lines, and treatment regime were similar (Table 1). As expected, patients with NAFLD had a higher BMI (P=0.004), and these patients were more likely to have non-squamous carcinoma (P=0.004).
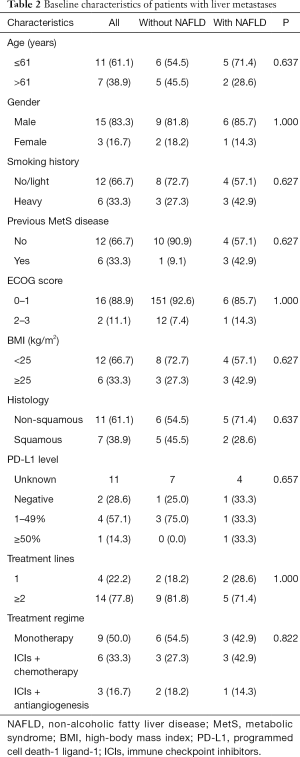
LMs were confirmed by imaging in 8.1% (n=18) of the patients (Table 2). In this subgroup, all patients were stage IV. Notably, there was no difference in BMI between the two groups.
Full table
Outcomes with ICIs-based treatment
Objective response
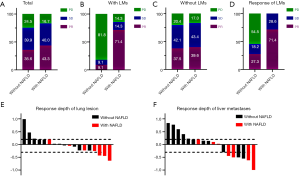
There was no significant difference in the response to ICI-based treatment between the patients with and without NAFLD (ORR 43.3% vs. 35.6%, P=0.289, DCR 83.3% vs. 75.5%, P=0.211) (Figure 2A). Notably, for patients with LMs, the ORR and DCR were dramatically higher in those with NAFLD (n=7) than in patients without NAFLD (n=11) (71.4% vs. 9.1%, P=0.013; 85.7% vs. 18.2%, P=0.013) (Figure 2B), while for patients with no LMs, the response was similar (ORR: 39.6% vs. 37.5%, P=0.784; DCR: 83.0% vs. 79.6%, P=0.689) (Figure 2C). In addition, DCR of LMs of patients with NAFLD was significantly higher compared to those without NAFLD (DCR: 42.9% vs. 0.0%, P=0.038) (Figure 2D). The response depth of lung primary lesions and LMs are shown in Figure 2E,F.
PFS
The median PFS of the entire cohort was 6.6 months (Figure 3A), and he median PFS of patients with and without NAFLD were similar (7.0 vs. 6.6 months, P=0.769, HR: 1.055, 95% CI: 0.738–1.507) (Figure 3B). However, in the subgroup of patients with LMs, the median PFS of patients with NAFLD was significantly longer than that of patients without NAFLD (5.1 vs. 2.1 months, P=0.014, HR: 0.244, 95% CI: 0.073–0.813), while no difference was observed in patients without LMs (7.0 vs. 7.3 months, P=0.476, HR: 1.146, 95% CI: 0.787–1.669) (Figure 3C,D). The PFS of each patient with LMs is shown in Figure 3E.
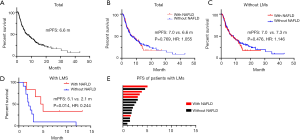
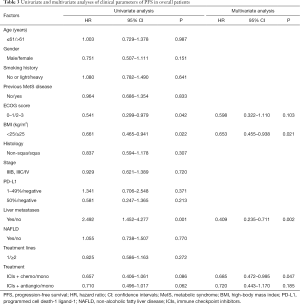
Based on univariate analysis for PFS among the entire cohort, liver metastasis was associated with decreased PFS (P=0.002, HR =2.492) and better ECOG was associated with increased PFS (P=0.042, HR =0.541). Furthermore, ICI combination therapy resulted in better PFS compared with ICI monotherapy. Meanwhile, our analysis indicated that low BMI (<25 kg/m2) associated with increased PFS (P=0.021). Based on multivariate analysis, BMI, liver metastasis, and ICI combined chemotherapy were associated with PFS (Table 3). Because of the limited sample size, we were unable to perform multivariate analysis for PFS among patients with LMs.
Full table
Discussion
In the current study, despite our initial expectations, NAFLD was not found to influence the efficacy of ICI-based treatment across the entire cohort of patients. Interestingly, in contrast with recent studies, our data suggested that low BMI was demonstrated to have survival benefit (Figure S1A) (3,4).

The effect of obesity in cancer patients is a topic of much controversy. Many studies have shown obesity to be associated with aggressive tumor biology (18). Wang et al. (19) found that obesity could result in immune dysfunction and tumor progression, although it also demonstrated greater anti-tumor efficacy and survival benefit after checkpoint blockade, which directly targets some of the pathways activated in obesity. However, data from the study of Donnelly et al. revealed a moderate but insignificant association between overweight or obese patients and better PFS in patients who received first line ICIs (20). Meanwhile, worse PFS was observed in obese patients who received non-first line ICI treatment (20), which is consistent with our results (Figure S1B,C). To date, the association between BMI and outcomes of ICI treatment in Chinese populations has been poorly reported, with the majority of related data originating from Western countries, where the median level of BMI is much higher.
Surprisingly, in the subgroup of patients with LMs, patients with NAFLD could derive more benefits, namely higher ORR and longer PFS, from ICI-based treatment. Moreover, for patients with LMs and NAFLD, the DCR of LMs was also better than those without NAFLD. Like obesity, NAFLD is commonly recognized as a risk factor for the onset of hepatic and extrahepatic tumors (21,22). Wolf et al. noted that non-alcoholic steatohepatitis activated intrahepatic CD8+ T cells and NKT cells, both of which are involved in the progression of hepatocellular carcinoma (HCC) (23). Ma et al. described that a ROS-dependent loss of hepatic CD4+ T helper cells, which was associated to obesity-related lipid dysregulation, led to increased hepatocarcinogenesis, whereas no effect was seen on CD8+ T cell numbers (24). Although there is much controversy surrounding the exact role of immune cells in NAFLD and further exploration of the involved mechanisms is needed, we know that adaptive and innate immune system are involved in the pathogenesis of NAFLD (7). Therefore, we speculated that the changes in composition in the liver regional immune microenvironment may exert a paradoxical impact on cancer in the same way obesity does. On the one hand, NAFLD induces immune dysfunction and tumor progression (11,22), but on the other, it may significantly impact the anti-tumor efficacy of ICI-based treatment, as indicated in our study.
Conclusions
In conclusion, our study indicated that NAFLD holds no clinical benefit for advanced NSCLC patients who undergo ICI-based treatments; but it is associated with improved outcomes in patients with LMs. However, this study was limited by its retrospective, single-center nature and the sample size of the subgroup of patients with LMs was small. Therefore, our results require further verification in larger, prospective studies. Further clarification of the mechanism of NAFLD in enhancing the effect of ICI-based treatment is also warranted.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (grant number: 81874036).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.04.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of Shanghai Pulmonary Hospital (No. 8187100412). Because of the study’s retrospective nature, the need for written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Russo A, Scilla KA, Rolfo C. Long-term efficacy of immune checkpoint inhibitors in advanced NSCLC: challenges and opportunities—a commentary of the 3-year follow-up of the KEYNOTE-001 trial. Transl Cancer Res 2019;8:S598-602. [Crossref]
- McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018;19:310-22. [Crossref] [PubMed]
- Kichenadasse G, Miners JO, Mangoni AA, et al. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019. [Epub ahead of print]. [PubMed]
- Valencia-Rodríguez A, Vera-Barajas A, Barranco-Fragoso B, et al. New insights into the association between non-alcoholic fatty liver disease and atherosclerosis. Ann Transl Med 2019;7:S300. [Crossref] [PubMed]
- Van Herck MA, Weyler J, Kwanten WJ, et al. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front Immunol 2019;10:82. [Crossref] [PubMed]
- Gadd VL, Skoien R, Powell EE, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014;59:1393-405. [Crossref] [PubMed]
- Inzaugarat ME, Ferreyra Solari NE, Billordo LA, et al. Altered phenotype and functionality of circulating immune cells characterize adult patients with nonalcoholic steatohepatitis. J Clin Immunol 2011;31:1120-30. [Crossref] [PubMed]
- Ma M, Duan R, Zhong H, et al. The Crosstalk between Fat Homeostasis and Liver Regional Immunity in NAFLD. J Immunol Res 2019;2019:3954890. [Crossref] [PubMed]
- Luo X, Li H, Ma L, et al. Expression of STING Is Increased in Liver Tissues From Patients With NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice. Gastroenterology 2018;155:1971-84.e4. [Crossref] [PubMed]
- Wu W, Liao H, Ye W, et al. Fatty liver is a risk factor for liver metastasis in Chinese patients with non-small cell lung cancer. PeerJ 2019;7:e6612. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Kanaji N, Tadokoro A, Watanabe N, et al. Association of specific metastatic organs with the prognosis and chemotherapeutic response in patients with advanced lung cancer. Respir Investig 2019;57:472-80. [Crossref] [PubMed]
- Salati M, Baldessari C, Cerbelli B, et al. Nivolumab in pretreated non-small cell lung cancer: continuing the immunolution. Transl Lung Cancer Res 2018;7:S91-4. [Crossref] [PubMed]
- Sridhar S, Paz-Ares L, Liu H, et al. Prognostic Significance of Liver Metastasis in Durvalumab-Treated Lung Cancer Patients. Clin Lung Cancer 2019;20:e601-8. [Crossref] [PubMed]
- Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res 2017;5:417-24. [Crossref] [PubMed]
- Kim D, Touros A, Kim WR. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin Liver Dis 2018;22:133-40. [Crossref] [PubMed]
- Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev 2015;95:727-48. [Crossref] [PubMed]
- Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019;25:141-51. [Crossref] [PubMed]
- Donnelly D, Bajaj S, Yu J, et al. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J Immunother Cancer 2019;7:222. [Crossref] [PubMed]
- Divella R, Mazzocca A, Daniele A, et al. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int J Biol Sci 2019;15:610-6. [Crossref] [PubMed]
- Massoud O, Charlton M. Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Clin Liver Dis 2018;22:201-11. [Crossref] [PubMed]
- Wolf MJ, Adili A, Piotrowitz K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014;26:549-64. [Crossref] [PubMed]
- Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531:253-7. [Crossref] [PubMed]


