
The prevalence and prognostic significance of estrogen receptor beta expression in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths across the world, with a yearly incidence of 1.82 million cases and 1.6 million deaths (1). The predominant form of the disease is non-small cell lung cancer (NSCLC), which accounts for 85–90% of cases (2). While treatment has improved significantly over the last 20 years, management of NSCLC is hampered by the advanced stage at which many patients present with disease, and a high likelihood of relapse following treatment (3). Modern advances in genomics allow development of therapies that target oncogenic driver mutations or translocations in genes such as EGFR (4) and ALK (5). More recently, immune checkpoint inhibitors have shown efficacy in the metastatic (6) and locally advanced settings (7). While these treatments are effective in the short term, relapse rates are high, and overall survival remains disappointingly low. We have a poor understanding of the factors that sustain tumor growth and development under metabolic conditions that would be toxic to normal cells.
An extensive body of epidemiological data highlights clear differences in the pathophysiology of lung cancer between men and women (8). For instance, while smoking is the primary cause of lung cancer in both sexes, never-smokers with cancer are significantly more likely to be female than male (9). Tumor histology is more likely to be adenocarcinoma in women (10), who also have generally better prognoses (11). Although these differences may be attributed to genetic and metabolic causes, further evidence implicates hormone signaling, particularly involving estrogen, in incidence and prognosis. In a study of 36,588 women, those receiving hormone replacement therapy with estrogen and progestin for 10 years or more were 50% more likely to develop lung cancer (12). In a large, randomized controlled trial conducted over a shorter period, women on hormone replacement therapy were almost twice as likely to die from lung cancer than in the placebo group (13). Notably, this increase in mortality was attenuated upon discontinuation of hormone replacement (14).
Although there are data to support a role for estrogen in the development and progression of lung cancer, the mechanism of action is unclear. The estrogen receptor (ER) protein is responsible for signal transduction events in response to estrogen and its analogues. The receptor exists in two variants that are expressed from different genes: estrogen receptor alpha (ERα) from the ESR1 gene, and estrogen receptor beta (ERβ) from ESR2. ERα is most commonly associated with breast, ovarian and endometrial cancers (15); however, its expression is most likely low or absent in NSCLC (16,17). Consequently, based on the hypothesis that estrogen acts directly on the tumor, several studies have assessed ERβ expression in NSCLC, and examined the association between expression and prognosis. There has been a complete lack of consensus among these studies: while some found that high ERβ expression was associated with poor prognosis (18,19), others found that low ERβ expression was a poor prognostic indicator (20-22), and others found no associations (23,24). There are at least two meta-analyses of these studies; one indicated that ERβ expression was a good prognostic marker (25), while the other found no prognostic value (26). Although most of these studies used immunohistochemistry to detect and quantify ERβ, they generally used different primary antibodies. This fact is significant, given that most of the commonly-used ERβ antibodies exhibit poor or no specificity for the protein (27,28). Another key variable is the method of analysis, which may be based on intensity of staining (29), proportion of stained cells (21,30), or a combination of both (19,31). In sum, the question of whether ERβ expression is associated with lung cancer outcome is significant but remains unresolved.
Our study was designed to test the hypothesis that ERβ expression is a prognostic factor in NSCLC. Given that there are prior studies which addressed this hypothesis, our goal was to resolve the inconsistencies of earlier studies with improved assay and analysis methods. We assessed the expression of ERβ in a tissue microarray (TMA) containing stages I–IV NSCLC. We took several measures to reduce methodological bias. First, we used an antibody (PPG5/10) whose specificity for ERβ has been extensively validated both by others (32) and by our own methods. This antibody recognizes only the full-length, transcriptionally-active splice variant of ERβ, and detects this protein in normal human lung epithelia (33). Second, we used high-sensitivity fluorescence immunohistochemistry to detect ERβ protein. Finally, we applied software-based image analysis for unbiased quantification of ERβ expression as a continuous variable. This allowed us to assess expression discretely in tumor, stroma, and subcellular compartments. The results suggested that the prognostic value of ERβ varied with the disease stage at presentation.
Methods
Patient selection
This study was approved by the Health Research Ethics Board of Alberta. All patients were diagnosed with NSCLC at the Tom Baker Cancer Centre in Calgary, Canada, between 2003 and 2006 (34). Clinical data were obtained retrospectively by chart review, and abstracted into the Glans-Look Lung Cancer Database (35). The 7th edition TNM classifications were used to stage all patients. A subset of patients received adjuvant treatment, which was chemotherapy, radiotherapy, or both.
TMA generation
The TMA for this study was as previously described (34), and was comprised of formalin-fixed, paraffin-embedded (FFPE) resected tumors and needle biopsies. Triplicate specimens were present for most patients. Slides bearing 4 µm-thick sections of the array were stained for ERβ, along with matched sections that were stained with isotype control antibodies. HeLa cell blocks were used as positive and negative controls (described below).
Protein expression controls
HeLa cells (CCL-2TM, ATCC, Manassas, VA, USA) were grown and maintained in Dulbecco’s Modified Eagle Medium (catalog number 11995073, Thermo Fisher Scientific, Waltham, MA, USA) containing fetal bovine serum (catalog number 10437010, 1:10, Thermo Fisher Scientific). Six million cells were seeded in a T175 flask and were transiently transfected with either 0.3, 0.4 or 0.5 µg/mL pcDNA-FLAG-ERβ plasmid (catalog number 35562, Addgene, Cambridge, MA, USA), or an empty vector expressing green fluorescent protein (pcDNA-GFP, kindly provided by Dr. Karl Riabowol, University of Calgary). The FLAG-ERβ expressed by this plasmid is the full-length, transcriptionally-active isoform of ERβ (ERβ isoform 1). Following expansion to 20 million cells per condition, cells were rinsed with PBS, dissociated with Versene (catalog number 15040-066, Thermo Fisher), and pelleted by centrifugation. Cell pellets were suspended in cold PBS, with 10% set aside for protein lysates and Western blot evaluation. The remaining cells were fixed in 10% neutral-buffered formalin (catalog number HT501128-4L, Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes, pelleted and resuspended in liquefied HistoGelTM (catalog number HG-4000-012, Thermo Fisher) at a concentration of 10 million cells per 100 µL. Samples were stored in 70% ethanol at 4 °C prior to processing and embedding the next day. Representative 0.6 mm cores from each cell block were incorporated into a testing array that served as an assay control for ERβ staining.
Fluorescence immunohistochemistry
TMA sections were deparaffinized and rehydrated, and heat-induced epitope retrieval with a citrate-based buffer (pH 6.0) was performed as described (35). To block endogenous peroxidase activity and eliminate non-specific antibody binding, peroxidase block (catalog number K4011, Dako, Mississauga, Canada) and Signal Stain® protein block (catalog number 8112L, Cell Signaling, Danvers, MA, USA) were applied in a hybridization chamber at room temperature. Slides were incubated in a humidified chamber overnight at 4 °C with either ERβ primary antibody (mouse monoclonal, clone PPG5/10, 1:500, Abcam, Cambridge, MA, USA) or mouse IgG1 isotype control (clone MG1-45, 1.0 mg/mL, Abcam). Staining with goat anti-mouse EnVision+ (catalog number K4007, Dako, Mississauga, Canada) secondary antibody was performed using a Dako Autostainer Link 48 followed by TSA Plus Cy5 signal amplification reagent (Perkin Elmer, Waltham, MA, USA) to visualize ERβ staining. The tumor epithelial compartment was identified by sequential staining with pan-cytokeratin (PCK) (rabbit polyclonal, catalog number Z0622, 1:100, Dako) and Alexa 555-conjugated anti-rabbit antibody catalog number (A21429, 1:200, Thermo Fisher) containing diamidino-2-phenylindole (DAPI) (catalog number D1306, 0.8 ng/µL. Thermo Fisher) to also visualize nuclei. Slides were cover slipped with ProLong Gold anti-fade mounting medium catalog number (P36934, Thermo Fisher) and stored at 4 °C until scanned.
Digital image acquisition and analysis
The workflow for image acquisition and software analysis is essentially as described in previous publications by our group (36-38) and others (39). Slides were digitized using an Aperio ScanScope FL slide scanner (Leica Biosystems, Concord, Ontario, Canada). An analysis algorithm was designed in the HALO image analysis software suite (version 1.94.392, Indica Labs, Corrales, NM, USA) (36). This algorithm identified cells based on nuclear expression of DAPI, and demarcated cell boundaries based on next-nucleus proximity and a maximum cellular radius of 5 µm. Cytoplasmic regions were defined as the difference, in pixels, between the whole-cell area and the DAPI-defined nuclear area. Cytokeratin-positive and -negative tissue regions defined the tumor and stroma, again as previously described (34). The software calculated the mean staining intensity of ERβ in cellular (whole cell, nucleus and cytoplasm) and tissue (tumor and stroma) compartments as the total fluorescent staining intensity in each compartment divided by the total cross-sectional area of the compartment, in each TMA spot. Backgrounds were calculated by applying the same algorithm to isotype control-stained sections of the same TMA; the highest mean staining intensity of isotype-stained sections was subtracted from values obtained in the presence of ERβ antibody. The resulting value, designated a HALO score, was measured for each of up to three TMA spots per patient sample. The mean HALO score from all TMA spots for each patient was used for statistical analysis.
Statistical analysis
Fisher’s exact test was used to analyze categorical data, and the Wilcoxon rank-sum test was used to assess continuous variables. Survival outcomes were analyzed using the Kaplan-Meier method, with the log-rank test to compare groups. Multivariate Cox-proportional hazards models were used to control for potential confounders. Patients were stratified into subgroups based on high and low HALO scores, using cut-points identified by X-Tile software (40). All statistical analyses were implemented using R (version 3.3.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
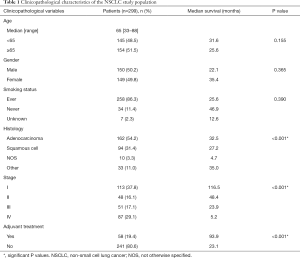
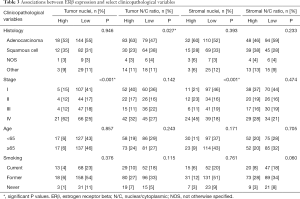
The study population consisted of 302 patients diagnosed with NSCLC, of whom 299 patients had ERβ expression data suitable for analysis. Their clinicopathologic characteristics are listed in Table 1. Most patients were diagnosed at stage I (37.8%), followed by stage IV (29.1%), stage III (17.1%) and stage II (16.1%). The percentages of patients presenting with adenocarcinomas or squamous cell carcinomas were, respectively: for stage I, 62.8% and 25.7%; stage II, 47.9% and 39.6%; stage III, 29.4% and 49.0%; stage IV, 60.9% and 24.1%. The proportions of male (50.2%) and female (49.8%) patients were almost equal. By stage at presentation, the percentages of patients that were male or female were, respectively, for stage I: 42.5% and 57.5%; stage II, 54.2% and 45.8%; stage III, 62.7% and 37.3%; stage IV, 50.6% and 49.4%. Only three patients received neoadjuvant therapy, which took the form of radiotherapy, chemoradiotherapy, or chemotherapy. In the patients who received adjuvant chemotherapy, there were significant differences in survival within groups, based on stage at diagnosis, receipt of adjuvant treatment, and histology (Table 1).
Full table
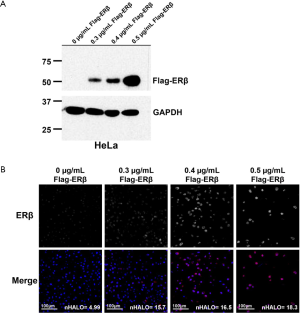
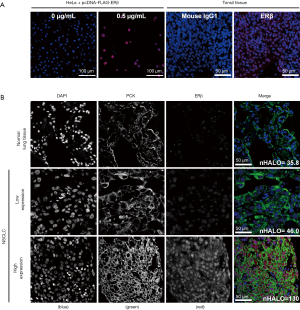
To verify the specificity of the primary antibody to ERβ, we created FFPE blocks from cells that over-expressed ERβ from a FLAG-tagged ESR2 plasmid. Western blotting (Figure S1A), and fluorescence immunohistochemistry on sections of the cell blocks (Figure S1B) were used to verify that only cells transfected with the ESR2 plasmid expressed ERβ. The HALO scores of ERβ-expressing cells increased with the amount of plasmid transfected, indicating that the assay was sensitive to different levels of protein expression. Representative images of ERβ staining in the NSCLC TMA are shown in Figure 1. The staining was predominantly nuclear, consistent with the localization of the transcriptionally-active ERβ isoform 1 detected by the PPG5/10 antibody (32).
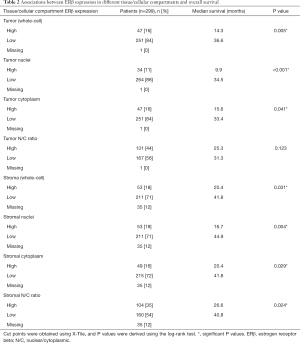
We assessed the correlation between ERβ expression, in different tissue and subcellular compartments of the NSCLC specimens, and overall survival. For the cohort as a whole, high ERβ expression correlated with shorter overall survival (Table 2). This association was evident with both nuclear and cytoplasmic expression of ERβ. Since the activity of certain signal transduction pathways may be regulated by subcellular localization of the active protein, rather than by level of expression, we also analyzed the nuclear/cytoplasmic (N/C) ratio of ERβ expression. A high N/C ratio of ERβ expression in the stroma, but not in the tumor, was associated with shorter overall survival (Table 2).
Full table
We examined associations between the clinicopathologic variables listed in Table 1 and ERβ expression in different tissue compartments. A subset of these analyses is shown in Table 3. There were no differences in ERβ expression between male and female patients, when stratified by either disease stage or tissue compartment. Remarkably, stage IV patients were more likely to express high levels of ERβ in tumor and stromal nuclei (P<0.001 in both cases). Stage IV patients were also more likely to have high ERβ expression in the tumor cytoplasm (P=0.030) and stromal cytoplasm (P=0.017). In stage IV patients, a high N/C ratio of ERβ in the tumor was associated with shorter overall survival (Figure 2A). Nuclear ERβ expression in stage IV patients was not prognostic (Figure 2B), nor was expression between male versus female patients (P=0.806).
Full table

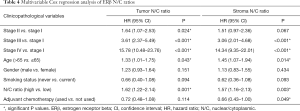
Multivariable Cox regression analyses were performed, using stage, age, histology, gender and smoking status as covariables. High N/C ratios of ERβ in the tumor and stroma were significantly associated with shorter overall survival (Table 4). Expression of ERβ in the nuclei was not associated with survival in either the tumor [hazard ratio (HR): 1.16, 95% confidence interval (CI): 0.77–1.76, P=0.480] or the stroma (HR: 1.15, 95% CI: 0.80–1.65, P=0.449). No other expression variables were associated with survival.
Full table
Discussion
In this study, we used fluorescence immunohistochemistry and software-based image analysis to detect and quantify ERβ expression in a NSCLC TMA. The tools employed in this work allowed us to quantify ERβ expression as a continuous variable, and to obtain data on expression from different tissue and cellular compartments. This analysis revealed that all patients expressed detectable ERβ, particularly in nuclei.
When the entire patient cohort was stratified by ERβ expression, high tumor and stromal expression were significantly associated with shorter overall survival; however, this was due to a strong association between high ERβ expression and stage IV disease. A high N/C ratio was significantly associated with shorter survival, when adjusted for stage in a multivariable model. There were no associations between ERβ expression and either gender or histology.
Since the primary antibody and analysis methods employed are significant determinants of results, there are few studies to which we can compare our own. The PPG5/10 monoclonal antibody was used in some prior studies to detect ERβ in lung cancer. The conclusions were that expression of ERβ isoform 1, which is the only isoform detected by the PPG5/10 antibody, was not associated with survival (29,31,41). Although the quantification methods were considerably different across the board, our results generally agree with those of these prior studies. Novel and unique to the present work, however, are the observations that ERβ expression predominated in advanced-stage NSCLC, and that the subcellular localization of ERβ is a significant prognostic factor. These observations warrant further investigation.
Although the mechanism underlying gender differences in the pathophysiology of lung cancer was partial impetus for this work, we observed no differences between male and female patients in ERβ expression. This does not invalidate the hypothesis that ERβ signaling may mediate differences in disease presentation and prognosis, since circulating estrogen levels are invariably higher in females. Instead, we posit that, should there be a tumor-intrinsic role for estrogen signaling in NSCLC, receptor targeting strategies would be effective in all patients.
The mechanisms underlying the key findings of this study are not clear. Higher ERβ expression levels in stage IV disease were apparent in the tumor as well as the surrounding stroma, and in both nuclei and cytoplasmic compartments. Similar results were reported previously (24). It is worth noting that the stage IV specimens were collected from needle biopsies, whereas stage I–III specimens were resected primary tumors. Differences in cold ischemic time and similar pre-analytical variables may explain differences in antigenicity of some proteins (42). There is no evidence that such effects may impact detectability of the ERβ protein, although we cannot rule out the possibility. We also observed that a high N/C ratio of ERβ expression was associated with shorter survival, again in stage IV patients. Given that this observation and the relevant statistical analysis were confined to this patient group (Figure 2), it must clearly be independent of any concerns about pre-analytical variables. It is possible that a high N/C ratio indicates that more cellular ERβ is present in its most biologically-relevant location—in the nucleus—and thus this ratio might serve as a proxy for activity. There are five known isoforms of ERβ, of which isoform 1, the predominant transcriptionally-active form, is the only one detectable with the PPG5/10 antibody (32). It is known that ERβ undergoes N/C shuttling (43), and it is suggested that the other isoforms may modulate the activity of isoform 1 (44); thus, a full understanding would require a careful analysis of the expression and distribution of the other isoforms.
In conclusion, our results suggest that high ERβ expression is associated with advanced NSCLC, and that preferential localization of the protein to the nucleus is associated with shorter overall survival. Since we observed no gender disparities in ERβ expression, therapeutic agents targeting this receptor may potentially be employed in male and female patients alike. Future studies should elucidate the signal transduction mechanisms that differentiate the α and β receptors, and guide downstream efforts to utilize this pathway in a clinical context.
Acknowledgments
Funding: This work was supported by donations to DGB through the University of Calgary to fund the creation and development of the Glans-Look Lung Cancer database. The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.34). HL reports other from Roche Canada outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of the Health Research Ethics Board of Alberta (HREBA.CC-16-0574) and and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Zakaria N, Satar NA, Abu Halim NH, et al. Targeting lung cancer stem cells: research and clinical impacts. Front Oncol 2017;7:80. [Crossref] [PubMed]
- Gadgeel SM. Personalized therapy of non-small cell lung cancer (NSCLC). Adv Exp Med Biol 2016;890:203-22. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Reck M, Heigener D, Reinmuth N. Immunotherapy for small-cell lung cancer: emerging evidence. Future Oncol 2016;12:931-43. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Rodriguez-Lara V, Hernandez-Martinez JM, Arrieta O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis 2018;10:482-97. [Crossref] [PubMed]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer 2007;7:778-90. [Crossref] [PubMed]
- Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol 2002;13:1087-93. [Crossref] [PubMed]
- Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005;128:370-81. [Crossref] [PubMed]
- Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol 2010;28:1540-6. [Crossref] [PubMed]
- Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009;374:1243-51. [Crossref] [PubMed]
- Chlebowski RT, Wakelee H, Pettinger M, et al. Estrogen plus progestin and lung cancer: follow-up of the women's health initiative randomized trial. Clin Lung Cancer 2016;17:10-7.e1. [Crossref] [PubMed]
- Chuffa LG, Lupi-Junior LA, Costa AB, et al. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids 2017;118:93-108. [Crossref] [PubMed]
- Kawai H. Estrogen receptors as the novel therapeutic biomarker in non-small cell lung cancer. World J Clin Oncol 2014;5:1020-7. [Crossref] [PubMed]
- Hsu LH, Chu NM, Kao SH. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci 2017. [Crossref] [PubMed]
- Skjefstad K, Grindstad T, Khanehkenari MR, et al. Prognostic relevance of estrogen receptor alpha, beta and aromatase expression in non-small cell lung cancer. Steroids 2016;113:5-13. [Crossref] [PubMed]
- Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res 2011;17:154-64. [Crossref] [PubMed]
- Chen XQ, Zheng LX, Li ZY, et al. Clinicopathological significance of oestrogen receptor expression in non-small cell lung cancer. J Int Med Res 2017;45:51-8. [Crossref] [PubMed]
- Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer 2008;59:88-94. [Crossref] [PubMed]
- Wu CT, Chang YL, Shih JY, et al. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg 2005;130:979-86. [Crossref] [PubMed]
- Aresti U, Carrera S, Iruarrizaga E, et al. Estrogen receptor 1 gene expression and its combination with estrogen receptor 2 or aromatase expression predicts survival in non-small cell lung cancer. PLoS One 2014;9:e109659. [Crossref] [PubMed]
- Monica V, Longo M, Felice B, et al. Role of hormone receptor expression in patients with advanced-stage lung cancer treated with chemotherapy. Clin Lung Cancer 2012;13:416-23. [Crossref] [PubMed]
- Luo Z, Wu R, Jiang Y, et al. Overexpression of estrogen receptor beta is a prognostic marker in non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med 2015;8:8686-97. [PubMed]
- Ma L, Zhan P, Liu Y, et al. Prognostic value of the expression of estrogen receptor beta in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2016;5:202-7. [Crossref] [PubMed]
- Skliris GP, Parkes AT, Limer JL, et al. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol 2002;197:155-62. [Crossref] [PubMed]
- Andersson S, Sundberg M, Pristovsek N, et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat Commun 2017;8:15840. [Crossref] [PubMed]
- Liu Z, Liao Y, Tang H, et al. The expression of estrogen receptors beta2, 5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine 2013;44:517-24. [Crossref] [PubMed]
- Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res 2005;11:7280-7. [Crossref] [PubMed]
- Mah V, Marquez D, Alavi M, et al. Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer 2011;74:318-25. [Crossref] [PubMed]
- Wu X, Subramaniam M, Negron V, et al. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem 2012;113:711-23. [Crossref] [PubMed]
- Hawse JR, Carter JM, Aspros KGM, et al. Optimized immunohistochemical detection of estrogen receptor beta using two validated monoclonal antibodies confirms its expression in normal and malignant breast tissues. Breast Cancer Res Treat 2020;179:241-9. [Crossref] [PubMed]
- Petersen LF, Klimowicz AC, Otsuka S, et al. Loss of tumour-specific ATM protein expression is an independent prognostic factor in early resected NSCLC. Oncotarget 2017;8:38326-36. [Crossref] [PubMed]
- Otsuka S, Klimowicz AC, Kopciuk K, et al. CXCR4 overexpression is associated with poor outcome in females diagnosed with stage IV non-small cell lung cancer. J Thorac Oncol 2011;6:1169-78. [Crossref] [PubMed]
- Enwere EK, Kornaga EN, Dean M, et al. Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical cancer. Mod Pathol 2017;30:577-86. [Crossref] [PubMed]
- Ho CK, Kornaga EN, Klimowicz AC, et al. Expression of DNA damage response proteins in cervical cancer patients treated with radical chemoradiotherapy. Gynecol Oncol 2017;145:176-84. [Crossref] [PubMed]
- Chanda A, Chan A, Deng L, et al. Identification of the SUMO E3 ligase PIAS1 as a potential survival biomarker in breast cancer. PLoS One 2017;12:e0177639. [Crossref] [PubMed]
- Martinez-Morilla S, McGuire J, Gaule P, et al. Quantitative assessment of PD-L1 as an analyte in immunohistochemistry diagnostic assays using a standardized cell line tissue microarray. Lab Invest 2020;100:4-15. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Toh CK, Ahmad B, Soong R, et al. Correlation between epidermal growth factor receptor mutations and expression of female hormone receptors in East-Asian lung adenocarcinomas. J Thorac Oncol 2010;5:17-22. [Crossref] [PubMed]
- Vassilakopoulou M, Parisi F, Siddiqui S, et al. Preanalytical variables and phosphoepitope expression in FFPE tissue: quantitative epitope assessment after variable cold ischemic time. Lab Invest 2015;95:334-41. [Crossref] [PubMed]
- Kassi E, Moutsatsou P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J Biomed Biotechnol 2010;2010:317452. [Crossref] [PubMed]
- Al-Bader M, Ford C, Al-Ayadhy B, et al. Analysis of estrogen receptor isoforms and variants in breast cancer cell lines. Exp Ther Med 2011;2:537-44. [Crossref] [PubMed]


