Nivolumab resulting in persistently elevated troponin levels despite clinical remission of myocarditis and myositis in a patient with malignant pleural mesothelioma: case report
Introduction
Malignant pleural mesothelioma (MPM) is a rare and aggressive malignancy, which is exclusively related to asbestos exposure. Although the Phase III MAPS study showed an improvement in median overall survival by 2.7 months with the addition of bevacizumab to cisplatin and pemetrexed chemotherapy, there have been no other significant recent advances (1). Furthermore, there is currently no approved second-line treatment for MPM.
Immune checkpoint inhibitors have altered the landscape and become the mainstay of treatment in many malignancies including melanoma and lung cancer (2,3). Despite being a disease with relatively low tumour mutational burden, there appears to be some benefit with immune checkpoint inhibition in MPM, with multiple phase II studies demonstrating benefit with nivolumab and pembrolizumab (2,4,5). The most promising data currently available demonstrated a 12-week disease control rate of 44% in patients on nivolumab and 50% of patients receiving nivolumab and ipilimumab, but the latter combination was associated with higher immune-related adverse events (irAEs) (6). While PD-L1 appears to enrich for patients most likely to respond to immune checkpoint inhibition, it lacks both sensitivity and specificity (2,6).
Although generally well tolerated, immune checkpoint inhibitors can critically affect multiple organs, including the rare risks of myositis and myocarditis, which could be fatal (7). We hereby report a patient with MPM from our institution who developed severe immune-related myositis and myocarditis following treatment with nivolumab therapy and achieved an impressive but short-lived anti-cancer response. This case report is in accordance with the CARE guidelines.
Case presentation
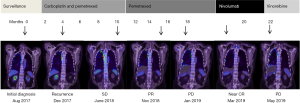
A 79-year-old retired carpet factory worker who presented with eight months history of dyspnea and chest wall pain was diagnosed with right-sided PD-L1 negative epithelioid MPM after undergoing VATS pleurodesis and biopsies in August 2017. After four months of post-operative surveillance, he became progressively more symptomatic secondary to progression of pleural disease (Figure 1). He completed six cycles of carboplatin and pemetrexed chemotherapy and continued on maintenance pemetrexed chemotherapy with stable disease. Six months later, on symptomatic progression of right pleural disease, nivolumab immunotherapy (3 mg/kg) was commenced.
Following two cycles of nivolumab, the patient presented to the emergency department with severe proximal limb and truncal weakness, dyspnea and generalized fatigue. The patient’s past medical history is significant for asbestos exposure, hypertension, dyslipidemia and stage 3 chronic kidney disease from benign prostatic hyperplasia. There was no prior history of autoimmune disease, although he had been on atorvastatin for longstanding dyslipidemia. He did not receive any recent influenza vaccination prior to immunotherapy. There was no relevant family history.
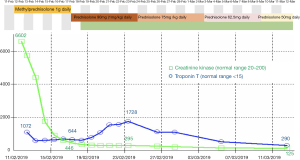
Subsequent testing revealed a creatine kinase (CK) level of 6,602 units/L (reference interval: 20–200 units/L) and high sensitivity troponin T (hsTnT) of 1,072 ng/L (reference interval <15 ng/L) (Figure 2). Relevant negative rheumatological panel markers included antinuclear antibody (ANA), extractable nuclear antigens (ENA), HMG-CoA reductase and Acetylcholine Receptor (AChR) antibodies. Electrocardiogram (ECG) showed no conduction abnormalities and bedside peak expiratory flow was normal. A clinical diagnosis of immune-mediated myositis and myocarditis was made.
The patient was commenced on 1,000 mg IV pulse methylprednisolone with daily clinical, ECG and biochemical monitoring. His proximal muscle weakness rapidly improved and a muscle biopsy performed showed necrotizing myositis. By day 7, the patient’s CK level significantly declined to 446 units/L. However, despite lack of symptoms of cardiac failure or myocarditis, and preserved left ventricular ejection fraction, hsTnT remained persistently high at 644 ng/L. In an attempt to understand the lack of concordance between his CK and hsTnT levels, troponin I was measured across three different assays. The results were consistent with our laboratory findings with elevated troponin I and raised NT-pro BNP levels, suggesting cardiac involvement (data not shown).
At day 8, the corticosteroid dose was weaned from 1,000 mg methylprednisolone to 1 mg/kg prednisolone given the resolution of clinical symptoms, despite failure of hsTnT to decline. Mycophenolate mofetil 1,000 mg twice daily was added as a steroid-sparing agent.
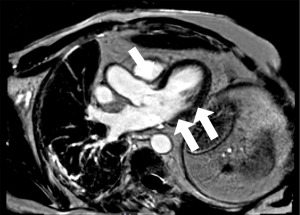
By day 13, hsTnT continued to escalate to 1,728 ng/L despite CK levels which continued to fall to 295 units/L. The patient remained clinically asymptomatic and no conduction abnormalities were detected. Cardiac MRI performed was consistent with myocarditis (Figure 3). The patient was subsequently discharged home despite persistently elevated serum hsTnT levels.
After 3 weeks, CK levels were within the low-normal range. In contrast, his hsTnT levels remained elevated, measuring 290 ng/L at 4 weeks, 200 ng/L at 8 weeks and 79 ng/L at 14 weeks. Prednisolone was weaned slowly to cessation over an 8-week period and the patient remains on mycophenolate mofetil to date. Restaging PET/CT scan following 2 cycles of nivolumab showed a near complete metabolic remission (Figure 1). Despite resolution of symptoms, the patient was not rechallenged with nivolumab due to subclinical myocarditis.
Unfortunately, radiological remission was not sustained. Repeat PET/CT scan 3 months following the last dose of nivolumab showed progression of pleural effusion and pleural nodules (Figure 1). As the patient remained clinically well, he was commenced on third-line treatment with vinorelbine chemotherapy which resulted in further disease progression. At time of reporting, the patient was currently being considered for further lines of chemotherapy.
Discussion
This report presents an unusual case of biochemical discordance with persistent markedly elevated troponin levels despite the absence of symptoms and normal CK level in a patient with myositis and myocarditis irAEs. To the best of our knowledge, this is the first case of immune related myocarditis and myositis in an MPM patient, who survived and achieved tumour response following 2 cycles of nivolumab.
Myositis and myocarditis irAEs are rare and while they are often floridly life-threatening, they can be difficult to diagnose and treat. The incidence of cardiac irAEs may be underestimated as ECGs, serial CK and troponin levels are not routinely undertaken in clinical practice. Patients typically present with severe limb girdle and axial weakness, with histological diagnosis of myonecrosis, as in the present case. Concomitant myocarditis is commonly reported with up to 40% of patients in small case series (8). The frequency of myocarditis through industry pharmacovigilance programs was reported at 0.09% (n=18/20, 594 patients) with 0.06% occurrence in single checkpoint inhibitors and 0.27% in combination therapy (9).
Both the onset time and the clinical presentation of cardiac irAEs are variable, with a median onset of 1 to 2 months but ranging between 1 to 13 cycles following treatment (7). In all cases, troponin and CK-MB were elevated, however notably ECG and transthoracic echocardiogram may be normal despite evidence of myocarditis on cardiac MRI and myocardial biopsy. Myocarditis irAEs can be life-threatening with 60% mortality rate despite early high dose corticosteroids (10). In those patients who survived, disease response is variable with 60% achieving partial response or stable disease and the rest experiencing disease progression post cessation of treatment (9).
Our patient had profound necrotizing myopathy with subclinical cardiac involvement. The diagnosis of myocarditis was made based on raised cardiac enzymes and cardiac MRI findings of myocardial inflammation. Although endomyocardial biopsy is the gold standard for myocarditis diagnosis, this is not routinely performed due to its invasive nature and risk of surgical complications (11). Notably, our patient developed worsening and persistent troponin rise for many weeks, despite the rapid clinical and biochemical resolution of his myositis, although he remained free of clinically evident cardiac symptoms. Previous case reports have shown that myocarditis presentation may be delayed, and patients with initial high troponin levels but a normal ECG may rapidly develop malignant ventricular arrhythmias (9).
One of the limitations of this study is that troponin rise may be produced by regenerating peripheral muscles cells, although infrequently of this magnitude. Serial cardiac MRI or endocardial biopsy may have allowed for better correlation with biochemical findings. Hughes et al. have reported elevated hsTnT but not troponin I to be a consequence of skeletal muscle breakdown and regeneration (12). In this case, the concordant presentation of both troponin I and T with cardiac MRI findings initially would strongly favour myocarditis, especially with exclusion of other syndromes such as absence of myasthenia gravis related autoantibodies and HMG-CoA reductase.
The extent of raised troponin levels is a prognostic factor of worse cardiovascular outcomes (11). In contrast, our patient survived with full resolution of symptoms and short-lived partial FDG PET/CT metabolic response despite significantly elevated troponin levels. Retrospective data on patients with advanced melanoma who developed irAEs after initial favourable disease response, demonstrated ongoing response despite cessation of immune checkpoint inhibitors (13). Skin irAEs such as vitiligo and rash, and rheumatoid irAEs appear to be a surrogate predictor of better treatment response, however, colitis, endocrinopathies or pneumonitis were not associated with survival advantage (14). In NSCLC, pneumonitis seemed to correlate with inferior overall survival (15).
The effect of myocarditis or myositis on tumour progression remains unknown, and treatment with immunosuppression is often intense and prolonged. There are currently no prospective clinical guidelines on subsequent management of cardiac irAEs and methods to assess treatment response following high dose corticosteroids. In our case, mycophenolate mofetil was selected based on efficacy reported in previous case reports (11).
Conclusions
The association of troponin level with myocardial damage in immune checkpoint inhibitor associated myocarditis is not clearly understood. It is important to acknowledge the lack of prospective studies in understanding the pathophysiology of immune checkpoint inhibitor related cardiac and rheumatic irAEs. More robust monitoring of serological activity including cardiac troponin levels in patients being treated with immune checkpoint inhibitors may help to identify subclinical myocarditis. Clinicians should be aware that myositis irAE can be associated with cardiac irAE and patients presenting with myositis, should be screened for cardiac involvement even in the absence of symptoms. Early diagnosis and treatment of cardiovascular irAEs are critical due to its associated high fatality rate. This is crucial especially in the current era as immune checkpoint inhibitors are increasingly utilized across multiple cancer types.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.05). AW reports personal fees from Eisai, personal fees from Ipsen, personal fees from Astellas Pharma, grants from Merck-Sorrono; SA reports personal fees and non-financial support from Astra Zeneca, personal fees from MSD, personal fees from Roche, personal fees from BMS, personal fees from Boehringer-Ingelheim, non-financial support from Pfizer; TJ reports personal fees from Astra Zeneca, personal fees from BMS, personal fees from Novartis, personal fees from MSD, personal fees from Merck-Sorrono, personal fees from Boehringer-Ingelheim, personal fees from Roche, personal fees from Pfizer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval Austin H2012/04888 and consent from patient acquired.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Garon EB, Hellman MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Ye L, Ma S, Robinson BW, et al. Immunotherapy strategies for mesothelioma - the role of tumor specific neoantigens in a new era of precision medicine. Expert Rev Respir Med 2019;13:181-92. [Crossref] [PubMed]
- Okada M, Kijima T, Aoe K, et al. Clinical Efficacy and Safety of Nivolumab: Results of a Multicenter, Open-label, Single-arm, Japanese Phase II study in Malignant Pleural Mesothelioma (MERIT). Clin Cancer Res 2019;25:5485-92. [Crossref] [PubMed]
- Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019;20:239-53. [Crossref] [PubMed]
- Agrawal N, Khunger A, Vachhani P, et al. Cardiac Toxicity Associated with Immune Checkpoint Inhibitors: Case Series and Review of the Literature. Case Rep Oncol 2019;12:260-76. [Crossref] [PubMed]
- Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985-94. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Heinzerling L, Ott PA, Husain AN, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer 2016;4:50. [Crossref] [PubMed]
- Ganatra S, Neilan TG. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018;23:879-86. [Crossref] [PubMed]
- Hughes M, Lilleker JB, Herrick AL, et al. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis 2015;74:795-8. [Crossref] [PubMed]
- Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol 2017;35:3807-14. [Crossref] [PubMed]
- Liew DFL, Leung JLY, Liu B, et al. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int J Rheum Dis 2019;22:297-302. [Crossref] [PubMed]
- Remon J, Reguart N, Auclin E, et al. Immune-Related Adverse Events and Outcomes in Patients with Advanced Non-Small Cell Lung Cancer: A Predictive Marker of Efficacy? J Thorac Oncol 2019;14:963-7. [Crossref] [PubMed]