
Hormone receptor expression correlates with EGFR gene mutation in lung cancer in patients with simultaneous primary breast cancer
Introduction
Both lung cancer (LC) and breast cancer (BC) are highly common malignancies around the world, ranking as the top two cancers in terms of incidence in female patients (1). With LC, especially non-small cell lung cancer (NSCLC), the majority of female patients show markedly different characteristics and genetic patterns compared to male cases (2). Although smoking is a prominent risk factor of LC, many female patients have never smoked and are diagnosed at a young age. Lung adenocarcinoma (ADC) comprises the vast majority of LC cases, while the incidence of squamous cell carcinoma (SCC) is considerably lower (3-5). Distinct pathology and molecular characteristics have been observed in these patients, among which the epidermal growth factor receptor (EGFR) pathway in NSCLC has been investigated most extensively (6). In fact, previous studies have shown that female ADC patients can have a higher EGFR mutation rate and show greater sensitivity to tyrosine kinase inhibitors (TKIs), such as gefitinib, compared with males (7,8). Such evidence indicates the underlying role of gender factor in NSCLC pathophysiology.
The levels of endogenous sex hormones have been proved to be associated with the development of several types of cancer such as BC, ovarian cancer, and endometrial cancer in females (9-11). Moreover, according to several prospective studies, increased sex-steroid hormones, through binding with hormone receptors [HRs, including estrogen receptor (ER) and progesterone receptor (PR)], can also influence cell biology and contribute to the development and progression of LC in females (12-14). Notably, in NSCLC, estrogen and progesterone signaling can interfere with EGFR-mediated signaling, especially ADC, through different mechanisms (14,15). In addition, EGFR expression alteration and gene mutation in response to estrogen or progesterone regulation in LC cells is a further reflection of the crosslink between the EGFR and ER/PR pathways (16-18). These basic findings indicate a potential correlation between EGFR expression status and ER/PR expression in NSCLC patients, especially females with ADC.
In clinical practice, a significant number of female LC patients with concurrent primary BC, which can be considered as double primary BC-LC cases, have been observed. It is not a rare phenomenon for a second primary tumor to occur after or while the patient is under the influence of treatment factors for the first primary tumor or for them to have specific risk factors and pathogenic agents in common (19). Previous studies found that coexistence of another primary malignancy in the lung with primary BC occurs more frequently in EGFR-mutant NSCLC type (20,21). Taking gender characteristics of LC into consideration, we suspected that, aside from radiotherapy, which could induce second primary LC in BC patients, aberrant expression and signaling of regional or circulatory female hormones could be a common or intersecting cause for the occurrence of simultaneous BC and LC (22).
Thus, to decipher the inner pathogenesis of double primary BC-LC, a total of 400 patients pathologically diagnosed as double BC-LC cases were enrolled in this study, specifically, in an effort to assess the association between HR expression and EGFR mutation status in lung tumor tissues via IHC and gene sequencing analysis. The expression of human epidermal growth factor receptor 2 (HER2) and the association with clinicopathologic factors were also analyzed. The expression status of BC-LC patients was further compared with LC-only patients to evaluate the similarities and differences between the two groups. Although several previous studies have also investigated such expression correlation, none have carried out comprehensive exploration of double primary BC-LC cases; this might actually be more instructive compared to a study solely focused on LC. Moreover, there is much inconsistency between the results from different laboratories, most likely due to use of different antibodies from different clones or the application of divergent staining protocols and standards for evaluation (23,24). With a sufficient sample size, our research can hopefully be one of the largest studies on double primary BC-LC and provide some direction for further investigation in the future.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-513).
Methods
Patients, samples and study design
A total of 400 female patients with double primary BC-LC who were diagnosed between 2010 and 2018 at Fudan University Shanghai Cancer Center were enrolled in this study. An additional 114 patients diagnosed as LC-only were also enrolled for comparison. All of the cases involved were pathologically confirmed. Written informed consent was obtained from each participant before samples were taken. The study was conducted with the approval of the Institutional Review Board of Fudan University Shanghai Cancer Center.
During the study, the clinical and demographics characteristics of all the patients were recorded in detail and aggregated for statistical analysis. Formalin-fixed paraffin-embedded (FFPE) sections with tumor content >80% were collected from 200 double primary BC-LC patients and 114 LC-only patients (as a contrast) for immunohistochemistry (IHC) analysis and EGFR mutation testing.
Confirmation of molecular classification and tumor staging
The recorded age in this study referred to the age at the time of initial pathological diagnosis. To distinguish primary tumor from a metastasis and prevent misclassification, the pathological diagnosis of primary LC and BC was strictly based on combined morphology and IHC evaluation. LC diagnosis referred to the 2015 World Health Organization (WHO) Classification of Tumors of the Lung (25). IHC markers were detected for LC subtyping. Based on the instructions of the IASLC (International Association for the Study of Lung Cancer) Pathology Committee, in cases where diagnosing ADC was challenging, we used TTF1 as a critical and essential marker combined with Napsin-A or other auxiliary markers, and p40 with p63 as an alternative. In cases where diagnosing SCC was difficult, CK5/6 or other markers were used (26). The IASLC 8th edition and AJCC 8th edition were used as the staging standards for primary LC and BC, respectively (27,28). Molecular subtypes of BC were classified into luminal A (ER and/or PR-positive, HER2-negative), luminal B (ER and/or PR-positive, HER2-positive), HER2 over-expressed (ER and PR-negative, HER2-positive), and triple-negative (ER, PR, and HER2-negative) (29).
Detection of EGFR mutations
Tissue specimens were obtained from patients during the process of pathological diagnosis or surgical procedures. Genomic DNA was extracted from the tumor tissues and purified using the TIANamp Genomic DNA Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions. As previously described, we amplified and sequenced all exons for the confirmation of EGFR mutation status (especially most common mutations such as exon 19 deletions delL747-A750, delE746-A750, and del L747-S752, and exon 21 point mutations L858R and L861Q) (6,7). The results were cross validated with TaqMan PCR assay analysis using specific primers, Taqman probes (GP Medical Technologies, Beijing, China) or State Food and Drug Administration (SFDA)-approved ADx EGFR Mutations Detection Kit (Amoy Diagnostics, Xiamen, China), based on the ARMS method and according to the manufacturers’ instructions (18,30).
IHC analysis
IHC was performed on tumor sections cut from paraffin block following standard procedures. The primary antibodies used in this study included monoclonal antibody 1D5 (dilution 1:150, No. M7047, DakoCytomation, Carpinteria, CA, USA) for ER, PgR636 (dilution 1:125, No. M3569, DakoCytomation) for PR, and anti-HER2 polyclonal antibody (dilution 1:175, No. A0485, DakoCytomation) for HER2/neu expression analysis. The immunohistochemical staining procedure was carried out in adherence with the manufacturer’s instructions. Breast tumor tissue samples known to contain the target molecules were used as a positive control. The replacement of the primary antibodies with phosphate-buffered saline was regarded as a negative control.
The staining quality of the specimens was evaluated based on several parameters including presence of positive reaction, staining intensity and cell proportion, cellular localization, and background staining level.
The American Society of Clinical Oncology/College Of American Pathologists (ASCO/CAP) guidelines were followed to interpret nuclear ER and PR staining status in BC, which were defined as positive for ER and PR expression in ≥1% of invasive breast carcinoma cells (31). For LC tumor tissues, the IHC staining was semi-quantitatively measured by the percentage of positive tumor cells. We used 10% as a threshold to make qualitative distinction. Generally, if ER or PR positive staining was detected in more than 10% of LC tumor cells, the case could be regarded as ER or PR positive (15). For those cases in an ambiguous state, two independent pathologists would be invited to assess the result. With HER2/neu expression, 0 to 3+ scoring was assessed according to membranous staining status, with 0 being regarded as HER2 negative and 1+ to 3+ as HER2 positive in lung tumor tissues (cutoff: 10%; specific scoring criteria as previously described) (32).
Statistical analysis
Standard frequency tabulations were used to summarize the patients’ clinicopathological information, EGFR mutation statuses, and IHC expression results. The correlation between molecule expression and the clinical and demographic variables of patients was evaluated using the χ2 test and Fisher’s exact test. A two-sided P<0.05 was considered to represent statistical significance. All statistical analyses in this study were performed using software SPSS (version 22.0, SPSS Inc., Chicago, IL, USA) and R (version 3.5).
Results
Basic characteristics of the study subjects
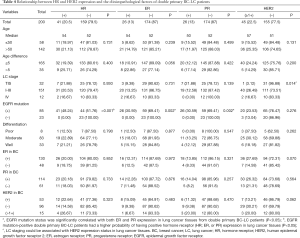
The clinical and demographic characteristics of a total of 400 female double primary BC-LC patients were recorded (Table 1). ADC accounted for 95.12% (370/389) of all LC cases, considerably more than SCC, neuroendocrine carcinoma, and adenosquamous carcinoma. Most LC patients with concurrent primary BC were diagnosed at an early stage and could be treated with surgery. EGFR mutation information was obtained from 154 of the BC-LC patients, among whom 74.68% (115/154) were confirmed to have EGFR mutation-positive LC tumor tissues. For the BC characteristics of the double primary BC-LC patients (Table 2), the invasive ductal carcinoma (IDC, 81.29%) and luminal subtypes (75.16%) were in the majority.
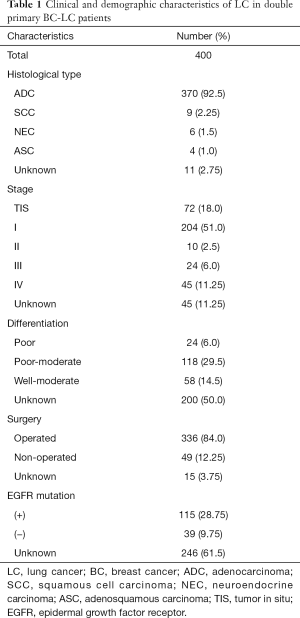
Full table
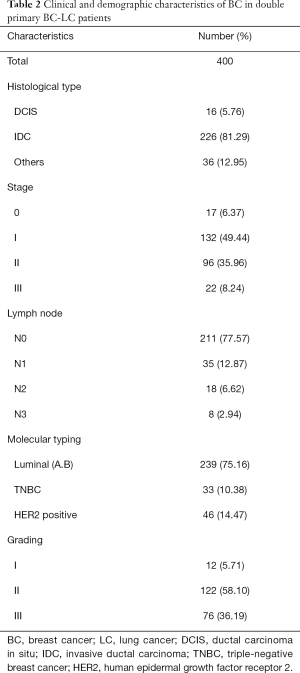
Full table
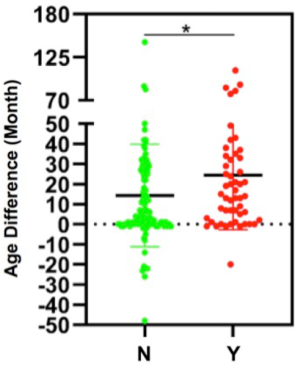
The average age of onset among the 400 BC-LC cases was 55 and 51 for LC and BC, respectively. According to the rules suggested by the International Agency for Research on Cancer (IARC), primary malignancies arising in different sites in the same individuals were classified into synchronous (diagnosed within a space of 6 months) or metachronous (more than 6 months) double primary cancers (33). In our study, synchronous and metachronous double primary cases accounted for 39.05% (66/169) and 60.95% (103/169) respectively among 169 BC-LC patients. For most metachronous double primary patients (n=93), LC was diagnosed later than BC (Figure 1).
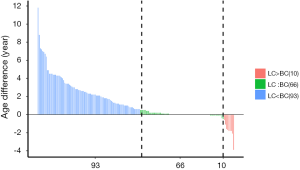
Subgroup statistics of double primary BC-LC patients
Basic clinical and demographic features including menstrual condition, family history of cancer, HR expression, and EGFR mutation status were compared between synchronous and metachronous double primary BC-LC patients. Based on the statistics available, the majority of (75.0%, 42/56) patients with a family history of tumors were diagnosed as metachronous-type. However, for those with no family history of tumors, the proportion with metachronous-type cancer was much lower (54.0%, 61/113). Family tumor history could be significantly correlated to the subtyping of synchronous or metachronous double primary cancers in this study (χ2=6.949, P=0.008, Table 3). We further compared the intervals of onset age between the two subgroups. Shorter intervals of onset age were observed in the double primary BC-LC patients who had no family tumor history, which indicates that for those patients without a hereditary background, the development of the two primary tumors could be attributed to the influence of causes such as hormone effect, rather than gene mutations (Figure S1). Apart from this, no significant difference was observed in other clinical factors between the synchronous and metachronous double primary BC-LC patients (P>0.05). HR expression and EGFR mutation status in LC tumor tissues appeared to have no relation to the onset age intervals of LC and BC in double primary patients (Table 3).
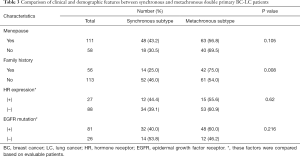
Full table
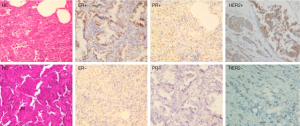
IHC staining results
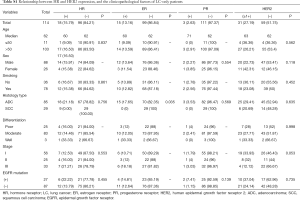
The expressions of ER, PR and HER2 in the tumor tissues were measured using IHC staining, and the representative examples of staining patterns are shown in Figure 2. For all the available data from 200 double primary BC-LC patients out of the 400 cases, the percentage of positive marker expression in LC tumor tissues was 13% (26/200) for ER, 13% (26/200) for PR, and 22.5% (45/200) for HER2. Among the 200 BC-LC patients, positive marker expression was 73.03% (130/178) for ER and 65.14% (114/175) for PR in corresponding BC tumor tissues (Table 4).
Full table
As well as those of the double primary cancer patients, the IHC results from a cohort of 114 LC-only patients were also analyzed. The positive rate of ER, PR, and HER2 expression in LC tumor tissues was 13.16% (15/114), 2.63% (3/114), and 34.44% (31/90), respectively (Table S1). Among the 114 LC patients, 25 female ADC cases were assessed separately, with the corresponding marker expression rates being 12% (3/25), 4% (1/25), and 47.8% (11/25) (Table S2).
Full table

Full table
HR positive referred to a positive test result for ER, PR, or both. Horizontal comparison revealed a relatively higher HR-positive rate in the double primary patients (20.5%, 41/200) in comparison with the LC-only patients (16.0%, 4/25) and female ADC patients (15.8%, 18/114).
Association between IHC markers and EGFR mutation status in double primary BC-LC patients.
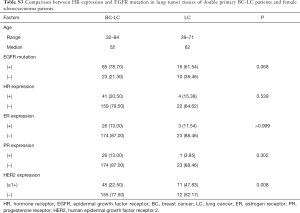
Table 4 describes the relationship between IHC marker expression statuses in LC tumor tissues and several clinicopathological characteristics, EGFR mutation status, and IHC staining results in BC tumor tissues from the double primary BC-LC patients. Among the BC-LC patients with positive EGFR mutation status, the positive rates for ER and PR expression in lung tumor tissues were both 30.59%. When EGFR mutations did not exist, both ER and PR could not be detected. The expression of HR (both ER and PR) in LC tumor tissues showed significant positive correlation with EGFR mutation in the BC-LC patients (P<0.05): higher HR-positive rate to higher EGFR mutation-positive rate correspondence. However, no significant difference was observed in HER2 and other IHC markers expression between mutated and wild-type EGFR status based on LC tumor tissue. All of the BC-LC patients with positive HR expression in lung tumor tissues simultaneously had positive EGFR mutation status at a rate of 100% (Tables 4,S2).
Comparative study in LC-only patients
The association between IHC markers and EGFR mutation in LC tumor tissues was also evaluated in 114 LC-only patients and 25 female ADC cases. ER, PR, or HER2 expression remained irrelevant to EGFR mutation status or other clinicopathological factors (P>0.05, Table S1 and Table S2) in LC-only patients. The results of IHC staining and PCR were further compared between the double primary BC-LC patients and single female LC patients (ADC type). HR expression and EGFR mutation in LC tumor tissues were relatively more common in the BC-LC patients compared to the LC-only cases. However, with P>0.05, larger study cohorts are needed to confirm this finding (Table S3).
Full table
Bioinformatics analysis of target genes
Lists of encoding genes of ER (ESR1 and ESR2), PR (PGR), and EGFR were input into DAVID Bioinformatics Resource 6.8 to perform gene functional clustering analysis. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) analysis revealed some probably shared pathways between EGFR and HR encoding genes, such as nitric-oxide synthase regulation, which could hint at the functional link of the markers (Table S4).

Full table
Discussion
Unlike BC, which is commonly known to be associated with sex hormones, hormone targeting in LC has not been considered before (9). However, increasing evidence has revealed that female LC patients exhibit distinctly different characteristics from males: they are often non-smokers, have a relatively younger age of onset, and develop ADC more often than their male counterparts (2). Above all, in female NSCLC patients with ADC, EGFR mutation is predominantly present, which indicates a potential relationship between EGFR mutation and sex-related factors, such as hormone levels (34,35). To decipher the inner mechanisms of this relationship, some researchers have attempted to identify a correlation between the expression of female HRs in lung tumor tissues with EGFR-mutation rate and clinical outcomes. However, the results have remained inconsistent, with a large range of HR expression frequencies among different studies, most likely caused by limited sample size or variations in criteria for detection (18,36,37).
The co-existence of primary EGFR-mutant LC and primary BC has been previously reported (20,38). According to a large population-based study, incidence rates of second primary NSCLC in BC patients are definitely higher than in the general population (39). Since both BC and LC represent gender-related attributes, it is reasonable to speculate that double primary BC-LC patients may exhibit more typical hormone-correlated molecular profiles. Therefore, to supplement previous findings, we enrolled a considerable number of patients diagnosed as double primary BC-LC at our institute to analyze their clinical and pathological characteristics and comprehensively verify the correlation between HRs expression and EGFR mutation in comparison to single NSCLC patients.
Echoing previous case reports and retrospective studies, lung ADC accounted for the vast majority (95.12%) of the 400 double primary BC-LC patients enrolled in this study. For most cases, LC was diagnosed after BC. In fact, advances in early detection and effective treatment have greatly improved BC prognosis and prolonged patients’ lifespan, which may inversely expose these patients to the risk of secondary primary malignancies, including LC (40). Difference in onset age was also analyzed among patients with and without family history of tumors. Interestingly, we observed that patients with no family tumor history might have shorter intervals of onset age of BC and LC and tended to be metachronous double primary BC-LC cases. We speculated that for these patients, genetic abnormality might have a considerable impact on the disease, which always occurred at different times. But for patients with a negative family history of cancer, the occurrence of two primary malignancies might be stimulated by some shared environmental factors, such as hormone levels, which might conversely contribute to synchronous double primary tumors.
In our study, IHC staining results were obtained from a total of 200 BC-LC patients. The positive expression rates for ER, PR (>10%), and HER2 (>1+) were 13%, 13%, and 22.5%, respectively. The expression of HRs showed no direct connection between lung and breast carcinoma tissues. Above all, we observed that HR expression in lung tumor tissues were distinctly correlated with the EGFR mutation rate in double primary BC-LC patients. Expression of both ER and PR was significantly higher in tumors with EGFR mutations (P<0.05). In contrast, HER2 seemed to have no connection with EGFR mutation status, with HER2 expression being undetectable in most lung tissues.
For further comparison, parallel expression analysis was conducted among the 114 patients diagnosed as LC alone. The positive rate of HR expression in lung tumor tissues was slightly lower in the LC-only patients than in those with simultaneous primary BC. Moreover, the correlation between HR expression and EGFR mutation was not similarly discovered in these patients as it was in the double primary BC-LC patients in our study. This phenomenon might reveal the potential difference in LC pathogenesis between patients with and without simultaneous primary BC. However, due to the limited sample size in our present study, more efforts are needed to decipher its inner mechanisms. Previous studies were mainly focused on single primary LC and the results were also extremely inconsistent between each of them (41). The reasons for this discrepancy cannot be confidently explained, which impairs a meaningful comparison of data. Our study was the first to analyze the relationship between HR expression and EGFR mutations in double primary BC-LC patients, which could add strength to the discovery of the role that sex hormones play in lung tumor tissue.
The crosstalk between hormone-mediated signaling and EGFR signaling has been postulated in NSCLC development (16). Expressed ER (both ER-α and ER-β) and PR might interact with EGFR mutations through some shared signaling pathways (42,43). For example, GPCR (G-protein-coupled receptor)-mediated transactivation of EGFRs could be triggered by estrogen via ERs to reveal the EGF-like effects of estrogen (44). According to Stabile et al., estrogen could induce the rapid release of EGFR ligands and modulate EGFR expression levels in LC cells, and the combination of ER antagonist and EGFR receptor TKI could assume antitumor effects by decreasing tumor cell proliferation and increasing apoptosis (16). In NSCLC cells, high aromatase mRNA expression was significantly correlated with EGFR mutation, leading to increased production of estradiol, ER pathway stimulation and tumor promotion (45). Estrogen could up-regulate the expression of osteopontin and then increase cell migration via activating the MEK/ERK signaling, a common downstream pathway mediated by EGFR activation (46). In addition, another downstream pathway of EGFR signaling- the PI3K/AKT signaling pathway could also be activated by estrogen, which facilitate the epithelial mesenchymal transition of lung cancer cells (47). Moreover, EGFR and ER may cooperate in the early activation of p42/p44 MAP kinase in NSCLC cells and promote tumor progression (15).
In NSCLC, the role of progesterone and its receptor has also been explored but remains controversial and may be quite different from estrogen. PR expression in NSCLC has often been reported to be associated with better prognosis, and progesterone treatment can significantly inhibit cell growth (48,49). Additionally, the protective effects of PR could be hormone independent, through interfering with EGFR signaling via PPD-SH3 interactions in PR-positive NSCLC (50). The biological meaning of the co-existence of HRs and EGFR mutation in NSCLC is still not fully elucidated. What’s more, given that little is known about the inner mechanisms of the occurrence of double primary BC-LC, our findings may offer the first step towards an improved understanding that previous studies could not.
Conclusions
In the present study, we explored the clinical characteristics of double primary BC-LC patients and subsequently discovered a positive correlation between ER, PR expression, and EGFR mutation in the lung tumor tissues of these patients, which might indicate the role of sex hormones in the development of primary LC. Despite a considerable number of double primary BC-LC patients having been analyzed, due to the incomplete clinical information and limited size of available tissue samples, there are still some limitations in our research that cannot be ignored. Moreover, further work is still required to enhance our understanding of the potential mechanisms behind this relationship.
Acknowledgments
This study was presented in part at the 20th annual meeting of World Conference on Lung Cancer (WCLC), Barcelona, Spain, Sep 7-10, 2019. The authors thank doctors from Fudan University Shanghai Cancer Center for the management of patients enrolled into this study.
Funding: This work was supported by National Key R&D Program of China (No. 2017YFC0907900/2017YFC0907904) and Wu Jieping Medical Foundation (No. 320.6750.18150).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-513
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-513
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-513). Dr. Hu reports grants from Ministry of Science and Technology of China, grants from Wu Jieping Medical Foundation, during the conduct of the study; Dr. Chang reports grants from Ministry of Science and Technology of China, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were approved by Institutional Review Boards of Fudan University Shanghai Cancer Center (No. 050432-4-1212B). Written informed consent was obtained from all of the patients involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Zhou F, Zhou C. Lung cancer in never smokers—the East Asian experience. Transl Lung Cancer Res 2018;7:450-63. [Crossref] [PubMed]
- Furrukh M. Tobacco Smoking and Lung Cancer: Perception-changing facts. Sultan Qaboos Univ Med J 2013;13:345-58. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Rochigneux P, Garon EB. Are lung adenocarcinoma mutations shaping the immune microenvironment? Transl Cancer Res 2018;7:S740-2. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Chen D, Chu T, Chang Q, et al. The relationship between preliminary efficacy and prognosis after first-line EGFR tyrosine kinase inhibitor (EGFR-TKI) treatment of advanced non-small cell lung cancer. Ann Transl Med 2019;7:195. [Crossref] [PubMed]
- Sieri S, Krogh V, Bolelli G, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev 2009;18:169-76. [Crossref] [PubMed]
- Andersen CL, Sikora MJ, Boisen MM, et al. Active Estrogen Receptor-alpha Signaling in Ovarian Cancer Models and Clinical Specimens. Clin Cancer Res 2017;23:3802-12. [Crossref] [PubMed]
- Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids 2015;99:8-10. [Crossref] [PubMed]
- Hsu LH, Chu NM, Kao SH. Estrogen, Estrogen Receptor and Lung Cancer. Int J Mol Sci 2017. [Crossref] [PubMed]
- Słowikowski BK, Lianeri M, Jagodzinski PP. Exploring estrogenic activity in lung cancer. Mol Biol Rep 2017;44:35-50. [Crossref] [PubMed]
- Kawprasertsri S, Pietras RJ, Marquez-Garban DC, et al. Progesterone receptor (PR) polyproline domain (PPD) mediates inhibition of epidermal growth factor receptor (EGFR) signaling in non-small cell lung cancer cells. Cancer Lett 2016;374:279-91. [Crossref] [PubMed]
- Márquez-Garbán DC, Chen HW, Fishbein MC, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007;72:135-43. [Crossref] [PubMed]
- Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 2005;65:1459-70. [Crossref] [PubMed]
- Rodriguez-Lara V, Hernandez-Martinez JM, Arrieta O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis 2018;10:482-97. [Crossref] [PubMed]
- Sun HB, Zheng Y, Ou W, et al. Association between hormone receptor expression and epidermal growth factor receptor mutation in patients operated on for non-small cell lung cancer. Ann Thorac Surg 2011;91:1562-7. [Crossref] [PubMed]
- Utada M, Ohno Y, Hori M, et al. Incidence of multiple primary cancers and interval between first and second primary cancers. Cancer Sci 2014;105:890-6. [Crossref] [PubMed]
- Moran T, Quiroga V, Cirauqui B, et al. A Single-Center Retrospective Study of Patients with Double Primary Cancers: Breast Cancer and EGFR-Mutant Non-Small Cell Lung Cancer. Oncol Res Treat 2019;42:107-14. [Crossref] [PubMed]
- Michalarea V, Calcasola M, Cane P, et al. EGFR-mutated lung cancer in Li-Fraumeni syndrome. Lung Cancer 2014;85:485-7. [Crossref] [PubMed]
- Kaufman EL, Jacobson JS, Hershman DL, et al. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol 2008;26:392-8. [Crossref] [PubMed]
- Dabbs DJ, Landreneau RJ, Liu Y, et al. Detection of estrogen receptor by immunohistochemistry in pulmonary adenocarcinoma. Ann Thorac Surg 2002;73:403-5; discussion 406. [Crossref] [PubMed]
- Raso MG, Behrens C, Herynk MH, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 2009;15:5359-68. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Yatabe Y, Dacic S, Borczuk AC, et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J Thorac Oncol 2019;14:377-407. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol 2018;25:1783-5.
- Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 2015;5:2929-43. [PubMed]
- Endo K, Konishi A, Sasaki H, et al. Epidermal growth factor receptor gene mutation in non-small cell lung cancer using highly sensitive and fast TaqMan PCR assay. Lung Cancer 2005;50:375-84. [Crossref] [PubMed]
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-95. [Crossref] [PubMed]
- Pellegrini C, Falleni M, Marchetti A, et al. HER-2/Neu alterations in non-small cell lung cancer: a comprehensive evaluation by real time reverse transcription-PCR, fluorescence in situ hybridization, and immunohistochemistry. Clin Cancer Res 2003;9:3645-52. [PubMed]
- Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open 2017;2:e000172. [Crossref] [PubMed]
- Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 2005;23:2513-20. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Berardi R, Morgese F, Santinelli A, et al. Hormonal receptors in lung adenocarcinoma: expression and difference in outcome by sex. Oncotarget 2016;7:82648-57. [Crossref] [PubMed]
- He Q, Zhang M, Zhang J, et al. Correlation between epidermal growth factor receptor mutations and nuclear expression of female hormone receptors in non-small cell lung cancer: a meta-analysis. J Thorac Dis 2015;7:1588-94. [PubMed]
- Jin B, Zhang S, Chuang X, et al. Breast cancer and synchronous multiple primary lung adenocarcinomas with heterogeneous mutations: a case report. BMC Cancer 2018;18:1138. [Crossref] [PubMed]
- Wang R, Yin Z, Liu L, et al. Second Primary Lung Cancer After Breast Cancer: A Population-Based Study of 6,269 Women. Front Oncol 2018;8:427. [Crossref] [PubMed]
- Mariotto AB, Rowland JH, Ries LA, et al. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev 2007;16:566-71. [Crossref] [PubMed]
- Di Nunno L, Larsson LG, Rinehart JJ, et al. Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med 2000;124:1467-70. [PubMed]
- Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 2003;17:309-17. [Crossref] [PubMed]
- Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 2002;62:2141-50. [PubMed]
- Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 2002;80:231-8. [Crossref] [PubMed]
- Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res 2008;14:4417-26. [Crossref] [PubMed]
- Hsu LH, Liu KJ, Tsai MF, et al. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci 2015;106:51-9. [Crossref] [PubMed]
- Zhao XZ, Liu Y, Zhou LJ, et al. Role of estrogen in lung cancer based on the estrogen receptor-epithelial mesenchymal transduction signaling pathways. Onco Targets Ther 2015;8:2849-63. [Crossref] [PubMed]
- Marquez-Garban DC, Mah V, Alavi M, et al. Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids 2011;76:910-20. [PubMed]
- Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res 2011;17:154-64. [Crossref] [PubMed]
- Abdellrazeq GS, Elnaggar MM, Osman HS, et al. Prevalence of Bovine Tuberculosis in Egyptian Cattle and the Standardization of the Interferon-gamma Assay as an Ancillary Test. Transbound Emerg Dis 2016;63:497-507. [Crossref] [PubMed]


