
Integrating clinical and biological prognostic biomarkers in patients with advanced NSCLC treated with immunotherapy: the DEMo score system
Introduction
Despite the improvement in overall survival (OS) of unselected advanced non-small cell lung cancer (NSCLC) patients treated with the immunotherapy (IO), biomarkers able to identify ideal candidate patients with adequate accuracy remain an unmet need. Continuous changes of the IO suggestion scenery, moving from second or further to first-line therapy or from single agent to the combination therapy with other companions [IO + chemotherapy (CHT), IO + CHT + bevacizumab or IO + IO], are among the main delay causes on finding the optimal biomarkers (1-8).
Up to today, the expression of the programmed-death ligand one (PD-L1) on tumor cells by immunohistochemistry (IHC) is the only approved biomarker. Indeed, despite patients expressing high levels of PD-L1 (≥50%) respond better to IO, some of them do not benefit from single agent IO. Conversely, a subgroup of patients with low PD-L1 (1–49%) still may benefit from IO alone, thus avoiding the toxicity added by other possibly companions such as chemotherapy. Another aspect includes the possibility to identify subjects with a non-negligible risk of early clinical failure (ICF) or hyper-progressive disease independently of PD-L1 expression (4-8).
According to literature, many attempts to discover predictive biomarkers outside PD-L1 have been made so far. The tumour mutation burden, CD8-positive tumour-infiltrating lymphocytes and immune gene signatures showed promising results as tissue biomarkers (9). However, tumor heterogeneity and the difficulties to obtain adequate tissue samples from aNSCLC patients, prompt for the use of scores systems based on clinical information or circulating biochemical and molecular factors. In this respect, markers such as the Lung Immune Prognostic Index (LIPI), based on the lactate dehydrogenase (LDH) levels and neutrophil-to-lymphocyte ratio (NLR), were created and associated with clinical outcome in IO settings (10,11). By adding information about the Eastern Cooperative Oncology Group Performance Status (ECOG-PS), sex, smoking habits and metastases sites, more complexes prognostic score systems such as Di Maio and EPSILoN were further generated (12-14). Among molecular biomarkers, the plasma microRNA signature classifier (MSC), developed for early lung cancer detection and reflecting an immunesuppressive host status (15,16), has recently shown its prognostic value also in aNSCLC patients treated with single agent IO (17).
The 3 markers were here compared and integrated in a unique score system called DEMo (Di Maio, EPSILoN, MSC). The aim of this prospective study was to assess if DEMo score is able to better categorize outcome of aNSCLC patients treated with IO and if the combination of these three biomarkers could improve the performance prediction compared to each single biomarker alone potentially helping clinical decision making.
Methods
Study population
From July 2015 to June 2019, we conducted a prospective observational study (Apollo, INT 22_15) enrolling 200 consecutive aNSCLC patients who received single-agent anti-PD-(L)1 inhibitors in 1L (n=70) or further-line therapy (n=130). Complete data were collected for both clinical scores. Whole blood samples were collected to assess LDH and NLR. The MSC test was prospectively assessed in plasma samples collected at baseline IO. Eligible patients fulfilled the following inclusion criteria: cytological/histological diagnosis of aNSCLC, patients (relapsed or stage IIIB to IV) that had received at least one infusion of anti PD-(L)1 single agent in 1L or further-line. This prospective study was conducted at the Fondazione IRCCS Istituto Nazionale Tumori of Milan in Italy and was accomplished in accordance with the Declaration of Helsinki, Good Clinical Practice and local ethical guideline. The present ongoing study was approved from local ethical committee and all included patients signed informed consent.
Treatment and response evaluation
IO was administered intravenously as monotherapy; Nivolumab was administered initially at a dose of 3 mg/kg and later, since May 2018 in Italy, at a fixed dose of 240 mg every 2 weeks (w); pembrolizumab at a dose of 2 mg/kg every 3 w in PD-L1 1–49% and at a fix dose of 200 mg in those patients with PD-L1 ≥50%; atezolizumab at a fixed dose of 1,200 mg every 3w and durvalumab at a dose 10 mg/kg every 2 w. Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1 criteria was used to assess tumor response (18). Response to IO was not valuable (NV) in patients who discontinued therapy after one cycle due to adverse effects or clinical deterioration. Therapy was continued until disease progression (PD), intolerable toxicity, withdrawal or death. Treatment beyond PD was allowed, if there was a clinical benefit according to clinician’s decision. Baseline radiological evaluations comprised a baseline total body computed tomography (TB-CT) scan, subsequently performed every 3–4 cycles or every 9–12 weeks, or whenever PD was clinically suspected.
Clinical and molecular markers
The Di Maio score combined clinico-pathological information such as sex, histology, ECOG-PS stage, uses of first-line platinum-based therapy and relative response (13,14). It stratified patients in three distinctive groups with a well-balanced cut-off along the range of values: <5, 5–9, >9 for the best (DiM_1), the intermediate (DiM_2) and the worst category (DiM_3), respectively (13,14).
EPSILoN combines clinical and biochemical information such as ECOG-PS, smoking status, presence of liver metastasis, lactate dehydrogenase (LDH) levels and the neutrophils-to-lymphocyte ratio (NLR) (19). Similarly to Di Maio, EPSILoN separated patients in three different prognostic categories: <1 for best (E_1), 1-–2 intermediate (E_2) and >2 worst category (E_3), respectively. The optimal cut-off for LDH and NLR values were determined using a statistic method enables calculation of both the cut-off value and its significance as previously described (19).
The plasma MSC test analyzed the reciprocal levels among 24 circulating microRNAs by quantitative reverse transcription PCR (RT-qPCR) as previously described (20). It stratified lung cancer patients in two main different prognostic groups, being MSC low/intermediate risk patients, with a better outcome compared to MSC high risk patients (17,21). Due to unspecific released of microRNAs in presence of cell lyses, highly heamolyzed plasma samples were undetermined for MSC and thus excluded from relative single marker analysis (22).
Data integration
A two-step data integration approach based on clinical evidence was adopted to generate the DEMo score system. In order to combine data from different sources into a single score, each group of patients identified by the 3 single markers received a score ranging from 1 to 3 according to their established prognostic value (12-14,21): score 1 for the DiM_1, E_1 and MSC intermediate/low risk groups with best prognosis (BP); score 2 for DiM_2, E_2 and MSC undetermined; score 3 for the DiM_3, E_3 and MSC high risk groups with worst prognosis (WP). The raw DEMo score system given by the sum of the three individual scores stratified patients in 7 risk groups with values ranging from 3 to 9. A second data elaboration step was then adopted to better evaluate the clinical utility of the DEMo score system. Indeed, 3 major DEMo groups were identified according to the balance between BP and WP groups according to the single markers: patients with DEMo score 3, exclusively composed by BP groups; patients with DEMo score from 4 to 6, where BP ≥ WP groups; and patients with DEMo score from 7 to 9, where BP < WP groups.
Statistical analysis
The endpoints were progression-free survival (PFS), overall survival (OS) and overall response rate (ORR) in strata of each single marker and the DEMo combined scores. OS was intended from the IO start date until death (event) or last follow-up (censored). Median PFS (mPFS) was considered from the IO start date until PD, death due to any cause (events), or last follow-up visit for patients alive without PD (censored). Survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test (23). Cox’s proportional hazards models were used to perform multivariate analyses. Overall response rate (ORR) was defined as the percentage of complete and partial response (R) among all patients. Patients with NV response to IO were excluded from ORR analysis.
The continuous variables were given as the median values and interquartile range (IQR). Interrater agreement of categorical variables was evaluated by the Cohen’s kappa statistic. All tests were two-sided, and P value <0.05 was considered statistically significant. Statistical analyses were performed using MEDCALC v.19.1.3 and PRISM–GraphPad v.5.02 software. Figure 1 was generated using Matlab script program v.R2019b.
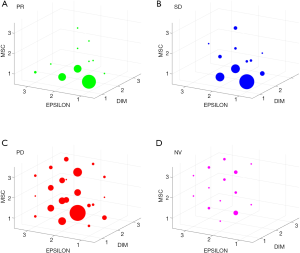
Results
Patients’ characteristics
Two hundred aNSCLC patients treated with anti-PD-(L)1 in 1L or further-line therapy were included in the analysis (Table S1). Most patients were male (65%) and smokers (79.5%) with median pack-year of 35 (IQR: 20–50). Median age was 67 years (range, 60–74 years) and 38% of patients were older than 70 years. Median ECOG-PS was 1 (range, 0–1) with an ECOG PS 2 in 14.5% of patients. All patients had a histological diagnosis of NSCLC (77% non-squamous and 23% squamous) and were EGFR non-mutated and ALK non-translocated. At baseline ICIs liver metastases were present 17.5% of patients. More than one third of patients (35%) received IO in 1L, while 65% received anti-PD-(L)1 therapy in second or further-lines. All 200 patients included in the study were assessable for survival analysis, but only 176 were evaluable for ORR. At the time of data cut-off (June 2019), 165 patients (82.5%) had disease progression and 142 patients had died (71%). The median follow-up for alive patients in the present cohort was 14.9 months.
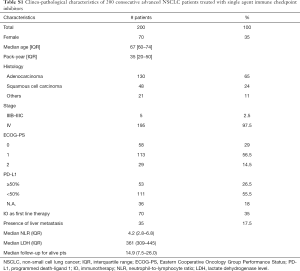
Full table
The single markers’ prognostic value (Figure S1)
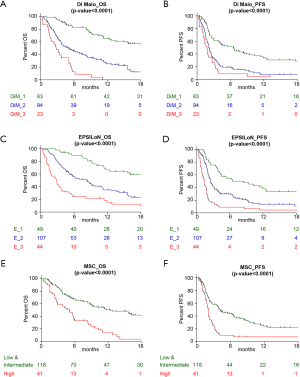
Both Di Maio and EPSILoN clinical scores divided patients into three categories with different prognosis: mOS was 21.3, 5.0 and 2.8 months for the 83 (41.5%) patients with DiM_1, the 94 (47%) DiM_2 and the 23 (11.5%) DiM_3, respectively (P<0.0001; Figure S1A). The mOS according to the EPSILoN score was 22.4 months for the 49 (24.5%) E_1, 8.3 months for the 107 (53.5%) E_2 and 2.9 months for the 44 (22%) E_3 (P<0.0001; Figure S1C). Adequate plasma samples to run the MSC test were available for 159 (79.5%) patients: mOS was 12.4 months in the group of 118 (59%) patients with MSC low or intermediate risk level and 4.7 months for the 41 (20.5%) patients with MSC high risk level (P<0.0001; Figure S1E). The remaining 41 (20.5%) patients had highly haemolyzed plasma samples and were thus not analyzable for the MSC test. Similar results were obtained when considering PFS as endpoint (Figure S1B,D,F).
Comparison of clinical and molecular markers
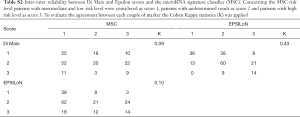
A score from 1 to 3 was attributed to each group of patients identified by the 3 individual markers, being 1 the group at best prognosis (BP) and 3 the groups at worst prognosis (WP). Analysis of the inter-rater reliability among the markers revealed a slight agreement when comparing both the Di Maio and EPSILoN scores vs. the MSC score (K≤0.10), while a moderate agreement (K=0.42) was observed when comparing Di Maio vs. EPSILoN (Table S2). Their independence was also confirmed by fitting Cox models for OS and PFS adjusted for the 3 markers. Results indicated that each marker maintained its prognostic significance while controlling for the other two: OS HR were 2.39 (95% CI: 1.82–3.16), 1.71 (95% CI: 1.30–2.26) and 1.42 (95% CI: 1.15–1.75) form Di Maio, EPSILoN and MSC, respectively (Table S3).
Full table
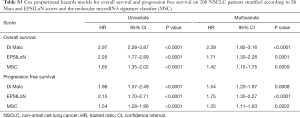
Full table
By stratifying patients according to response to IO, 13 out of 36 (36%) responder (R) and 14 out of 48 (39%) patients with stable disease (SD) were in the BP group for the 3 markers simultaneously, but no one was in the WP group for more than 1 marker (Figure 1A,B). Conversely, among patients with progressive disease (PD) only 2 out of 116 (2%) were included in all the 3 BP group, while 17 (15%) were in the WP group for at least 2 markers (Figure 1C). Similarly, when considering the 24 patients who discontinued therapy after one cycle due to adverse effects or clinical deterioration, no one patient had 3 markers with score 1, while 8 (33%) had at least 2 markers with score 3 (Figure 1D).
The integrated DEMo prognostic score system
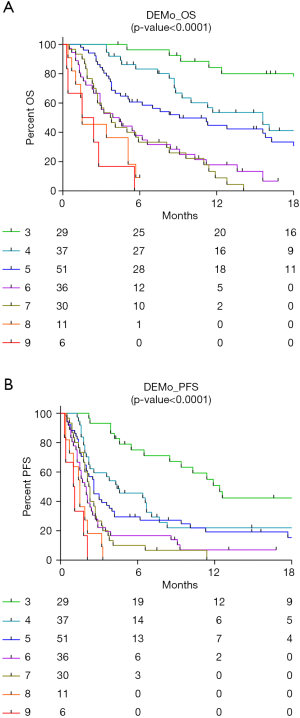
The raw DEMo score system generated by the integration of the 3 single prognostic markers, stratified patients in 7 groups with a score ranging from 3 to 9. Throughout these groups, mOS had a trend ranging from 29.7 to 1.5 months (P<0.0001; Figure 2A) and mPFS from 12.4 to 1.1 months (P<0.0001; Figure 2B).
For further analysis, patients were combined in three major DEMo groups according to the balance between BP and WP groups for the 3 single markers: 29 patients were included in the DEMo score 3 group, patients with DEMo scores from 4 to 6 were 136 and 35 patients had DEMo scores from 7 to 9. Hazard ratio (HR) from multivariate OS analysis adjusted for age, sex, smoking status, ECOG-PS and line of therapy were 5.37 (95% CI: 1.55–18.62), 3.14 (95% CI: 1.48–6.66), 2.13 (95% CI: 1.36–3.34) and 13.13 (95% CI: 3.85–44.81) when comparing the two extreme prognostic groups according to Di Maio, EPSILoN, MSC and DEMo, respectively (Table 1). Comparable results in terms of PFS were obtained for each marker, being the HR in strata of the DEMo score system the highest one (HR: 7.46; 95% CI: 2.61–21.29).
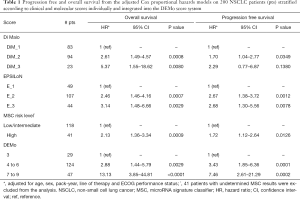
Full table
When considering the ORR as end-point (Table S4), analysis between the two extreme prognostic groups resulted in a relative risk of response (RR) of 0.19 (0.03–1.33), 0.28 (0.09–0.88) and 0.33 (0.11–1.02) for Di Maio, EPSILoN and MSC markers, respectively. On the other hand, the corresponding analysis comparing ORR in the 35 patients with DEMo scores 7 to 9 versus the 29 patients within the DEMo score 3 group resulted in a RR =0.06 (95% CI: 0.01–0.46).
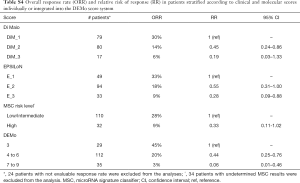
Full table
DEMo models according to PD-L1 status
PD-L1 status was available in 164 (82%) patients of the present series. In order to evaluate the clinical utility of the 3 single markers and the DEMo score system according to PD-L1 expression levels, two distinct models were adopted. Model_1 was defined to identify PD-L1 ≥50% patients (n=53) who less benefit from single agent IO by comparing patients in the WP group vs. all other patients. The mOS and mPFS were respectively 2.4 and 1.9 months for the 13 (25%) aNSCLC patients with DEMo scores 7 to 9, while not reached and 11.4 months for the other 40 patients (Figure 3A,B). Conversely, in order to identify PD-L1 <50% patients (n=111) who may still benefit of single agent IO, the Model_2 compared patients in the BP group vs. all other patients. According to Model_2, a not reached mOS and a 10.3 months mPFS for the 12 (11%) aNSCLC patients with DEMo score 3 were compared to the 5.7 months mOS (P=0.0005) and 2.1 months mPFS (P<0.0001) of the remaining 99 patients with higher scores (Figure 3C,D).
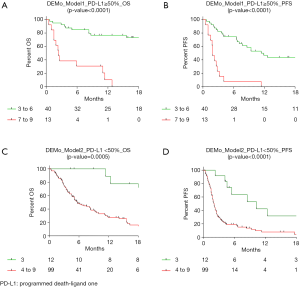
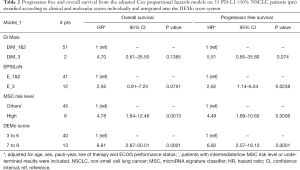
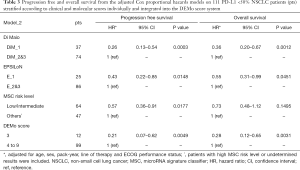
In PD-L1 ≥50% patients, the Model_1 adjusted HR from multivariate analysis for OS was 4.70 (95% CI: 0.61–35.5), 2.56 (95% CI: 0.91–7.23), 4.78 (95% CI: 1.84–12.46) and 8.81 (95% CI: 2.87–20.01) for Di Maio, EPSILoN, MSC and DEMo, respectively (Table 2). By stratifying PD-L1 <50% patients according to Model_2, the adjusted OS HR for the BP groups were 0.26 (95% CI: 0.13–0.54) for Di Maio, 0.43 (95% CI: 0.22–0.85) for EPSILoN, 0.57 (95% CI: 0.36–0.91) for MSC and 0.21 (95% CI: 0.07–0.62) for DEMo (Table 3). Similar results were obtained when considering PFS (Tables 2 and 3).
Full table
Full table
Discussion
The DEMo score system resulting from the combination of three different bio/markers: the Di Maio and EPSILoN clinical scores and the MSC molecular test. As prognostic marker in aNSCLC patients treated with IO, DEMo was able to perform better compared to each single bio/marker alone.
The DiM prognostic score was initially developed (13) and validated (14) in patients with aNSCLC receiving 2L CHT and included only clinical features: ECOG-PS, sex, histology, stage, uses of platinum-based therapy at 1L and response to 1L. Authors concluded that patients in the worst category could have a slight chance to benefit from active anti-tumour treatments and probably best supportive care might be the best choice (14). Here we reported that the prognostic value of Di Maio was maintained also in patients receiving IO by identifying a subgroup with very short life expectancy. Similarly, the EPSILoN score, composed by both clinical (ECOG PS, smoking history and presence of liver metastases) and biochemical (NLR and LDH) factors, was trained in a cohort of aNSCLC patients treated with CHT and was recently validated in aNSCLC patients treated with IO (12).
The plasma MSC molecular test was developed for early lung cancer detection in samples collected from LC patients and healthy volunteers enrolled in low-dose computed tomography screening trials (24). It stratified LC patients in 3 levels according to the risk to develop lung cancer in its aggressive form (16). The MSC diagnostic (high and intermediate vs. low risk level) and prognostic (high vs. intermediate and low risk level) value was independent to tumor characteristics such as stage, histology or mutational load (12). On the other hand, changes in circulating microRNA levels composing the MSC were associated to a protumorigenic and immunosuppressive phenotype of stromal and haematopoietic lineages such as fibroblasts, macrophages, polymorphonuclear and endothelial cells (15,25).
Combining and integrating different markers in a unique composite score could potentially ameliorate patient selection. The LIPI score developed by Mezquita et al. (11 trials and 3,987 pts with aNSCLC) was created using two variables (NLR and LDH). This score was able to separate 3 different survival groups (good, intermediate and poor) in aNSCLC patients treated with IO compared to chemo- (10) and target-therapy (11) (controls arms); A recent paper on 21 different cancer types and 7,187 patients using anti-PD-1/PD-L1 agents showed that among 36 (multiomics prediction) the three top variables which better correlate with ORR were estimated CD8+ T-cell abundance, TMB and high PD-L1 gene expression (26). Here, the DEMo score system divided patients in 7 categories based on the combination of the three prognostic bio/markers previously reported (12-14,21). Each marker maintained its prognostic value in the present series by identifying BP and WP groups of aNSCLC patients treated with IO single agent. Patients included in the 3 BP groups (DEMo score 3) most benefit from IO. Conversely, patients included in more WP than BP groups (DEMo scores 7, 8 and 9) less benefit from IO single agent.
In order to assess the clinical utility of the DEMo score system, a sub-group analysis adding information on PD-L1 status was also performed. Indeed, considering the results of recent clinical trials such as Keynote-189 and checkmate-227 (27,28), PD-L1 expression would drive therapy selection in daily practice (i.e., in our country, still, patients with high PD-L1 expression undergo pembrolizumab alone as first line therapy, while patients with non-squamous NSCLC and low PD-L1 expression perform CHT + IO, IO remain still a second line for patients with squamous-NSCLC and low PD-L1). With the idea to identify PD-L1 strong positive aNSCLC patients who could probably benefit more from combination therapy (CHT + IO or CHT + IO + anti-angiogenic drugs), the DEMo Model_1 was developed. In this context, DEMo identified a 25% of patients who poorly benefit from single agent IO. On the contrary, among patients with low PD-L1 expression the DEMo Model_2 identified a small percentage of patients (11%) who could still benefit from single-agent IO and could thus avoid unnecessary toxicity from the combo-therapy.
The main limitation of our study was given by the impossibility to analyze a control arm, and thus to evaluate if DEMo could also be considered a predictive marker. In fact, when the Apollo prospective study started in 2015, the vast majority of aNSCLC patients underwent IO or were included in double blind clinical trials testing IO or IO-based combination therapies. The remaining aNSCLC patients treated with CHT were those excluded from clinical trials as not in compliance with enrolment criteria such as age or ECOG PS and, for the same reasons, cannot be used as control arm. Furthermore, only by testing the DEMo score system in aNSCLC patients treated with the recently approved IO combination therapies, we will understand the real clinical utility of such a test.
Conclusions
We created this composite clinical-molecular combined score called DEMo in order to test its prognostic utility in aNSCLC patients treated with first or further-line IO. Results indicated that DEMo identifies those patients who better or who are less likely to benefit from IO single-agent. To our knowledge, this is the first prospective exploratory study which tried to combine clinical, biochemical and molecular markers in order to create a composite score which take into account baseline characteristics that potentially predicts survival outcomes in IO regimens. Moreover, we successfully apply different DEMo models in appropriate clinical settings in order to potentially improve the patients’ selection given by PD-L1 status.
Given the prospective nature of the study, here we integrated previously identified markers composed of several different features. Nevertheless, in the era of big data, artificial intelligence should be used as an efficient approach to help clinicians to manage lot amounts of data from different sources in order to create better predictive models to choose the most efficient IO-based therapy and sequence.
Acknowledgments
We would like to thank our nurse assistant: Anna Maria Leone, Giuliano Molino and Antonia Martinetti for the support with blood sample collection.
Funding: This work was supported by Italian Ministry of Health (GR-2016-02361849) and investigator grants No. 18812 from the Italian Association for Cancer Research.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-231). AP reports grants from Italian Association for Cancer Research during the conduct of the study; personal fees from Roche, AstraZeneca and BMS outside the submitted work; in addition, AP has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. CP reports grants from Italian Association for Cancer Research during the conduct of the study; personal fees from BMS and MSD outside the submitted work; in addition, CP has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. GLR reports grants from Italian Association for Cancer Research during the conduct of the study; personal fees from BMS, MSD and AstraZeneca outside the submitted work; in addition, GLR has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. DS reports grants from Italian Association for Cancer Research during the conduct of the study; personal fees from BMS, AstraZeneca and Boehringer Ingelheim outside the submitted work; in addition, DS has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. RF reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, RF has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. GG reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, GG has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. ADT reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, ADT has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. GV reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, GV has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. MB reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, Marta B has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. RL reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, RL has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. BT reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, BT has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. FT reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, FT has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. VT has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. GS reports grants from Italian Association for Cancer Research during the conduct of the study; in addition, GS has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. MCG reports grants from Italian Association for Cancer Research during the conduct of the study; personal fees from Roche, AstraZeneca, BMS, MSD, International GmbH, Boehringer Ingelheim Italia S.P.A, Celgene, Eli Lilly, Ignyta, Incyte, Inivata, MedImmune, Novartis, Pfizer, Roche and Takeda outside the submitted work; in addition, MCG has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. Mattia B reports grants from Italian Ministry of Health during the conduct of the study; in addition, Mattia B has a patent IT1406672 licensed to Gensignia LS, a patent IT1403685 licensed to Gensignia LS, and a patent IT1406866 licensed to Gensignia LS. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethic committee of the Fondazione IRCCS Istituto Nazionale Tumori of Milan in Italy (No. INT22_15) and all included patients signed informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Califano R, Kerr K, Morgan RD, et al. Immune Checkpoint Blockade: A New Era for Non-Small Cell Lung Cancer. Curr Oncol Rep 2016;18:59. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev 2019;75:39-51. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1- positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Garassino MC, Cho BC, Kim JH, et al. ATLANTIC Investigators. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer 2019;106:144-59. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Kazandjian D, Gong Y, Keegan P, et al. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:1481-5. [Crossref] [PubMed]
- Prelaj A, Ferrara R, Rebuzzi SE, et al. EPSILoN: A Prognostic Score for Immunotherapy in Advanced Non-Small-Cell Lung Cancer: A Validation Cohort. Cancers (Basel) 2019;11:1954. [Crossref] [PubMed]
- Di Maio M, Lama N, Morabito A, et al. Clinical assessment of patients with advanced non-small-cell lung cancer eligible for second-line chemotherapy: a prognostic score from individual data of nine randomised trials. Eur J Cancer 2010;46:735-43. [Crossref] [PubMed]
- Di Maio M, Krzakowski M, Fougeray R, et al. Prognostic score for second-line chemotherapy of advanced non-small-cell lung cancer: external validation in a phase III trial comparing vinflunine with docetaxel. Lung Cancer 2012;77:116-20. [Crossref] [PubMed]
- Fortunato O, Borzi C, Milione M, et al. Circulating mir-320a promotes immunosuppressive macrophages M2 phenotype associated with lung cancer risk. Int J Cancer 2019;144:2746-61. [Crossref] [PubMed]
- Sozzi G, Boeri M, Rossi M, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol 2014;32:768-73. [Crossref] [PubMed]
- Boeri M, Milione M, Proto C, et al. Circulating miRNAs and PD-L1 Tumor Expression Are Associated with Survival in Advanced NSCLC Patients Treated with Immunotherapy: a Prospective Study. Clin Cancer Res 2019;25:2166-73. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics & Data Analysis 1999;30:253-70. [Crossref]
- Mensah M, Borzi C, Verri C, et al. MicroRNA Based Liquid Biopsy: The Experience of the Plasma miRNA Signature Classifier (MSC) for Lung Cancer Screening. J Vis Exp 2017.56326. [PubMed]
- Verri C, Borzi C, Holscher T, et al. Mutational Profile from Targeted NGS Predicts Survival in LDCT Screening-Detected Lung Cancers. J Thorac Oncol 2017;12:922-31. [Crossref] [PubMed]
- Fortunato O, Boeri M, Verri C, et al. Assessment of circulating microRNAs in plasma of lung cancer patients. Molecules 2014;19:3038-54. [Crossref] [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- Boeri M, Verri C, Conte D, et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A 2011;108:3713-8. [Crossref] [PubMed]
- Andriani F, Majorini MT, Mano M, et al. MiR-16 regulates the pro-tumorigenic potential of lung fibroblasts through the inhibition of HGF production in an FGFR-1- and MEK1-dependent manner. J Hematol Oncol 2018;11:45. [Crossref] [PubMed]
- Lee JS, Ruppin E. Multiomics Prediction of Response Rates to Therapies to Inhibit Programmed Cell Death 1 and Programmed Cell Death 1 Ligand 1. JAMA Oncol 2019;5:1614-8. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]


