Improved overall survival following tyrosine kinase inhibitor treatment in advanced or metastatic non-small-cell lung cancer—the Holy Grail in cancer treatment?
The introduction of the epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) gefitinib (Iressa®, AstraZeneca, UK), erlotinib (Tarceva®, Roche, Switzerland), and afatinib (Giotrif®, Boehringer Ingelheim, Germany) and the anaplastic lymphoma kinase (ALK) inhibitors crizotinib (Xalkori®, Pfizer, USA) and ceritinib (Zykladia®, Novartis, Switzerland) represent the most important innovations in non-small-cell lung cancer (NSCLC) treatment over the past ten years (1). By targeting the main pathways of NSCLC signal transduction, these drugs significantly improved progression-free survival (PFS) and quality of life in a highly selected subgroup of NSCLC (harbouring EGFR mutations), sparing them from toxic chemotherapy approaches. However, for the vast majority of patients platinum-based chemotherapy remains the only potential treatment and has led to significantly improved survival outcomes with a “plateau” of about 10-11 months median survival (2). Subsequently, significant advances have been made with the introduction of pemetrexed, especially against the non-squamous cell subtype. The addition of this agent led to a further improvement in survival to 12-13 months (3) and up to 14 months with the introduction of maintenance therapy (4).
Maintenance therapy is a treatment strategy that has been investigated extensively in NSCLC and has been the subject of considerable recent debate. Options for maintenance include continuing the initial combination chemotherapy regimen, continuing only single agent chemotherapy (‘continuation maintenance’) or introducing a new agent (‘switch’ maintenance therapy). Therapies that have been studied in this setting in randomized trials to date include chemotherapy, molecularly targeted agents and immunotherapy approaches (5).
The outstanding results of the JMEN study proved that maintenance of pemetrexed (for patients with tumours of non-squamous histology) significantly improved the overall survival (OS) in advanced NSCLC patients was a proof of principle (6). Subsequently, the results of the SATURN study also showed a significant prolongation of PFS and OS with maintenance erlotinib (for patients with stable disease) compared with placebo (7). Despite considerable controversy, it has become an acceptable treatment paradigm and both drugs are approved for maintenance therapy of advanced NSCLC patients in Europe (EMA) and the USA (FDA) and this has certainly shifted the pendulum towards maintenance therapy.
Zhang and colleagues (8) first presented results from the INFORM trial evaluating gefitinib in the maintenance setting in 2012 (8). In this large phase III multicentre, double-blind trial patients (Asian ethnic origin, n=296) with stage IIIb or IV NSCLC after four cycles of platinum-based doublet chemotherapy were randomized either to placebo or maintenance therapy with gefitinib (250 mg/d) until progression or unacceptable toxic effects. Primary endpoint was PFS as assessed in the intent-to-treat population, whereas OS was a secondary endpoint. Assessment of PFS according to the tumour EGFR mutation status was also a pre-planned exploratory objective [highlighted in a previous editorial in this journal by Dempke (9)].
Median duration of treatment was 148 [49-467] days with gefitinib and 73 [42-127] days with placebo. PFS was significantly longer with gefitinib than that with placebo [median PFS 4.8 (95% CI: 3.2-8.5) vs. 2.6 (1.6-2.8) months; hazard ratio 0.42; 95% CI: 0.33-0.55; P<0.0001]. OS did not differ between both treatment groups [hazard ratio 0.84; 95% CI: 0.62-1.14; P=0.26; median OS 18.7 (95% CI: 15.6-22.2) vs. 16.9 (14.5-19.0) months]. Moreover, the greatest PFS benefit with gefitinib was found in the subgroup positive for EGFR mutations [hazard ratio 0.17; 95% CI: 0.07-0.42; median PFS 16.6 (9.4-22.7) vs. 2.8 (1.3-4.1) months].
In a most recently published update of the INFORM trial OS results were detailed (10). The median duration of follow-up for OS was 17.83 months (95% CI: 15.43-20.23). At the time of data cut-off for OS (June 17, 2014), 230 patients (78%) had died. In the subgroup positive for EGFR mutation, a higher OS was observed in patients treated with gefitinib than the placebo arm (HR 0.39; 95% CI: 0.15-0.97; P=0.036; median OS 46.87 vs. 20.97 months). In contrast, there was no significant difference in OS for gefitinib vs. placebo in patients negative for EGFR mutations (HR 1.27; 95% CI: 0.7-2.3; P=0.431; median OS 10.9 vs. 14.0 months). In the subgroup with unknown EGFR mutation, OS was numerically but not statistically longer with gefitinib vs. placebo (HR 0.92; 95% CI: 0.68-1.25; P=0.603; median OS 20.6 vs. 16.8 months). However, it is worth noting that a large proportion of patients (73%) had insufficient tumour samples to perform a mutation analysis.
Targeted therapies are currently being evaluated in a variety of treatment settings in NSCLC and novel strategies of disrupting tyrosine kinase-controlled pathways have been investigated. However, almost all of the recently reported trials have failed to improve OS for which there may be several key reasons.
Firstly, without a validated biomarker, specific subgroups of patients who are more likely to respond cannot be selected. Furthermore, the redundancy in tyrosine kinase-triggered pathways leads to primary and secondary resistance to an agent that targets a specific signal transduction cascade; as a result, agents that target multiple pathways are currently under investigation. Finally, it is unlikely that any TKI could achieve complete inhibition of its target(s), which may result in reduced but not completely abrogated signalling (11). Moreover, the reasons that TKIs have failed to improve survival when added to chemotherapy remain far from clear. A possible potential mechanism for the lack of synergy between these agents and chemotherapy may be the G1 phase cell-cycle arrest caused by TKIs, which then may interfere with the cell cycle-dependent cytotoxicity of chemotherapy (12).
The question remains whether the benefit of targeted therapy for NSCLC may be best defined by PFS since in this regard published data are still inconclusive. Truly, PFS is regarded as a good predictor for improved OS (and is independent of subsequent treatment), but OS is acknowledged as the key clinical outcome in the treatment of advanced NSCLC. All large previous randomized phase III trials assessing first-line treatment demonstrated a significantly higher response rate and longer PFS in patients treated with first- and second-generation EGFR-TKIs, including gefitinib, erlotinib, and afaftinib than in patients treated with standard platinum-based combination chemotherapy. Although these trials met their primary endpoint with significantly longer PFS, no significant difference was observed in terms of OS. However, no restrictions were imposed on treatment after the end of protocol therapy in any of these trials and the majority of patients in the control arm received EGFR-TKI therapy at least once (Table 1).
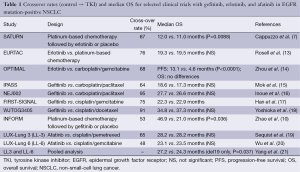
Full table
None of these randomized trials has yet demonstrated a statistically significant improvement with these TKIs in terms of OS, which is of course the strongest endpoint for clinical research in oncology, in a condition of no effective treatment afterwards. When effective treatment is given as post therapy, it will be difficult to distinguish the treatment effect of original and subsequent treatments because differences in OS are potentially confounded by crossover, and a relevant number of patients assigned to chemotherapy arms received TKIs as second- or third-line treatment after disease progression (Table 1). Intuitively, the high proportion of crossover may extend the benefit associated with the administration of TKIs to patients assigned to the control arm, and its “salvage”-effect may compensate for the relevant differences in PFS of first-line treatment consistently demonstrated in all TKI trials.
However, a most recently published joint analysis of the LUX-Lung trials 3 and 6 revealed that afatinib prolonged survival of patients with NSCLC with common EGFR mutations compared with standard chemotherapy by a median of 3 (27.3-24.3) months, significantly reducing the risk of death by 19% (HR =0.81, CI =0.66-0.99; P=0.037). The most pronounced reduction in risk of death, by 41% (HR =0.59, CI =0.45-0.77; P<0.001), was noted for patients whose tumors have the most common type of EGFR mutation (namely deletion in exon 19), which is present in approximately 48% with an EGFR mutation. For patients with the exon 21 (L8585R) mutation, there was no impact on OS (HR =1.25, CI =0.92-1.71; P=0.160) (21). From a methodological point of view, subgroup and post-hoc analyses can be informative, but should be interpreted with caution since PFS was chosen as the primary endpoint in both trials
Moreover, crossover was high for afatinib and erlotinib, and very high for gefitinib in all studies (Table 1) making the statistical power for analysis of OS very low (22,23).
In conclusion, the updated results of the INFORM trial clearly do not support the routine use of gefitinib for maintenance therapy as standard of care in NSCLC patients with advanced or metastatic NSCLC following treatment with platinum-based chemotherapy. However, to our knowledge the INFORM study is the first randomized clinical trial that shows a significant OS benefit in the EGFR mutation-positive population following maintenance therapy with gefitinib as compared to placebo. It remains to be seen whether further exploration of this treatment strategy will confirm these promising data.
Acknowledgements
Disclosures: Klaus Fenchel and Ludger Sellmann declare no conflicts of interest. Wolfram Dempke is an employee of AstraZeneca Ltd (UK).
References
- Dempke WC. Targeted therapy for NSCLC–a double-edged sword? Anticancer Res 2015;35:2503-12. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [PubMed]
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432-40. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [PubMed]
- Dempke WC. Gefitinib in non-small-cell lung cancer—an old lesson new re-visited. Transl Lung Cancer Res 2013;2:435-8. [PubMed]
- Zhao H, Fan Y, Ma S, et al. Final overall survival results from a phase III, randomised, placebo-controlled, parallel-group study of gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804). J Thorac Oncol 2015;10:655-64. [PubMed]
- Aggarwal C, Somaiah N, Simon G. Antiangiogenic agents in the management of non-small cell lung cancer: where do we stand now and where are we headed? Cancer Biol Ther 2012;13:247-63. [PubMed]
- Sharma SV, Fischbach MA, Haber DA, et al. "Oncogenic shock": explaining oncogene addiction through differential signal attenuation. Clin Cancer Res 2006;12:4392s-5s. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [PubMed]
- Yoshioka H, Mitsudomi T, Morita S, et al. Final overall survival results of WJTOG3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harbouring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2014;32:5s.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [PubMed]
- Rossi A, Di Maio M. LUX-Lung: determining the best EGFR inhibitor in NSCLC? Lancet Oncol 2015;16:118-9. [PubMed]
- Hotta K, Suzuki E, Di Maio M, et al. Progression-free survival and overall survival in phase III trials of molecular-targeted agents in advanced non-small-cell lung cancer. Lung Cancer 2013;79:20-6. [PubMed]


