
Challenges and issues surrounding the use for translational research of human samples obtained during the COVID-19 pandemic from lung cancer patients
Introduction
The pandemic due to infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), so called the coronavirus disease 2019 (COVID-19) pandemic, has probably led to a slowdown in the sanitary taking care in patients with many different diseases, including cancers. One of the obvious consequences was the reorganization over only a few days in hospitals and university medical centers of different departments providing patient care, including the emergency services and critical care units. The almost daily improvement in the knowledge into the epidemiology rapidly modified the work of other clinical and hospital departments such as those involved in care of patients with cancer, patients who are very susceptible to infection with SARS-CoV-2 (1-3). Notably, the current pandemic requires unusual allocation of healthcare resources which may negatively impact the care of patients with thoracic cancer that continue to require urgent medical attention. Certain hospitals providing thoracic surgery and managing surgical lung specimens were sometimes limited by local decisions leading to decrease their workload or to adhere to some national recommendations. Some of these decisions were made to meet the medical and paramedical needs for the more or less urgent care of hospitalized patients with COVID-19 and were based on the need to reprogram surgery considered to be less urgent. The real impact of COVID-19 on surgery in thoracic oncology is difficult to estimate at present but since the beginning of the pandemic there is most certainly a decrease in activity, which differs according to the institution and the local, regional and national epidemiology of this viral infection.
In the context of the COVID-19 pandemic, the collection of biological samples (fluids and tissues) for research programs in oncology and the management of the biobanks are directly concerned, notably in the area of thoracic oncology. In fact, it is certainly in this latest domain that the work of the biobank should be the most affected. Moreover, it should be pointed out that a number of studies, while controversial for some, indicated that the population of patients with lung cancer contracts COVID-19 more often than the general population (1-4).
Information concerning the degree of contagiousness of SARS-CoV-2, notably in a laboratory of pathology, requires that good practice in hygiene and security be adopted for management of biological samples. Knowing that uncertainty exists concerning the presence and viability of the virus in different samples this information may lead to new recommendations, particularly for the collection and use of tissues and fluids for research in thoracic oncology.
This review deals with the present challenges that pathologists as well as biobankers must urgently face during the COVID-19 pandemic for the management of samples for research in thoracic oncology. It also presents the possible requirements that those requesting samples may express in the near future.
Management of samples in pathology laboratories and potential recommendations
Recent publications show that SARS-CoV-2 is present and viable for at least several hours on different surfaces and in the air in aerosols and in droplets expired by patients (5,6). The capacity of different coronaviruses to reside and survive on different surfaces (paper, cardboard, aluminum, plastic, sponges, surgical masques, latex gloves, etc.) has been reported, particularly after the first epidemics of SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV) and more recently during the SARS-CoV-2 pandemic (7-9). SARS-CoV-2 can be detected in a number of organs, tissues, cells and biological fluid and is most certainly viable in a number of non-fixed biological samples (10). This is particularly true for samples of tissues and cells obtained from the departments of lung pathology [biopsies, bronchoalveolar lavage (BAL), bronchial aspirations and smears, bronchial swabs, endobronchial ultrasound cytology, pleural fluid, etc.] and thoracic surgery (surgical specimens from the lung, pleural and mediastinal samples). The contagiousness characteristic of non-fixed samples sent to the pathology laboratories is probably a reality, despite the fact that no case of infection of staff handling one of these samples in a pathology laboratory has been reported to date. The handling of surgical samples or of non-fixed biopsies of a patient with a suspected lung cancer, in particular for frozen section examination requires selected, oriented and multiple sampling performed by the pathologist. Frozen tissue can be infected and these sections in a cryostat can be made by the technician. Cytological lung samples are often transferred fresh to the pathology laboratory and must be immediately treated by the technician, notably centrifuged (11,12). All this work requires the adoption and adherence of high precautions concerning hygiene and security as described below, while following a certain number of recommendations put forward very recently at an international level.
The general precautions to follow in handling all biological samples in a pathology laboratory are of course those already applied before the COVID-19 pandemic. However, it is important to reiterate these precautions and to train all the staff but also to reinforce protection and decontamination procedures (7). Aside from the bronchopulmonary tree, the presence of SARS-CoV-2 or its RNA has been detected in many other organs including those from the head and neck area, the digestive tract, the central nervous system and the kidney until now, and more exceptionally in feces and blood (10,13-22). Other organs are potentially concerned, including the heart and blood vessels containing cells expressing angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor which was initially identified on bronchial cells (23-25). However, the virus may not be detected in other biological samples (26). Nonetheless, the regulations concerning hygiene and security must, in principle, be applied to the handling by the clinical pathology laboratory of all non-fixed tissue and cellular samples (11,12). In addition, the COVID-19 pandemic also changed suddenly the way molecular pathology laboratory worked (27,28). In this context, the biosafety rules need to be applied to molecular pathology work. So, some institutions have prioritized fully automated technologies to limit long hands on time which is usually mandatory for next generation sequencing approach (27,28). This is of strong interest when working with non-fixed samples, notably with liquid biopsies. This also applies to autopsies and all suspicions of infection with SARS-CoV-2 which require an autopsy have to be done following the biosafety level 3 (BSL3) guidelines as for other coronaviruses (29-33).
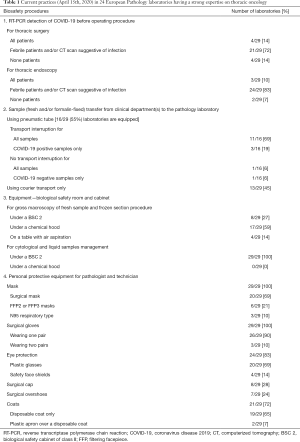
Thus, a number of comments can be made for the management of human samples in the period of COVID-19 pandemic, notably in a laboratory of pathology. The regulations for good practice in the handling of tissue and cellular samples in pathology have already existed for a long time since all non-fixed samples are potentially infectious, disregarding the pathology. So pathologists and technicians are well aware of the possible infectious nature of some cancer samples infected with pathogens such as hepatitis B or C viruses, Human Immunodeficiency virus, mycobacteria or others pathogens such as respiratory viral agents, just to mention a few, all of which are known to be virulent when present in human samples. Thus, the question as to whether new precautions need to be established and adopted in the context of the COVID-19 pandemic can be asked. Since the start of this pandemic and as the weeks pass more and more is learnt about the SARS-CoV-2 and its potential degree of virulence, which is higher than initially suspected. Thus, the survival of the virus in air and contamination of the air, even at a distance of several meters from a subject to another one, is possible and may be greater than for other identified coronaviruses (7). As consequences, this requires that the pneumatic tubes existing in a number of pathology laboratories should not be used. These pneumatic tubes transport samples from the sites of endoscopy or from operating rooms to the pathology laboratories. It is in fact a rapid, economical and simple way of optimizing the traceability of the transport and of better mastering the time of cold ischemia of the non-fixed sample (34-36). Moreover, many hospitals have prohibited the use of formalin in surgical departments that are not equipped with chemical hoods. These pneumatic tubes can be used to transport tissues, cells and biological fluids including blood. One of the consequences of the international recommendations that take into consideration the COVID-19 pandemic is the suppression of the use of pneumatic tubes to transfer non-fixed samples from patients infected with SARS-CoV-2 to the pathology laboratories (37-40). It is noteworthy that this restriction was already applied during the previous SARS-CoV-1 epidemic (41). A preliminary survey made on the 15th of April 2020 in 15 French pathology laboratories (headed by pathologist experts in thoracic oncology belonging to the “PATTERN” group) and in 14 pathology laboratories of different countries [USA, Spain, Italy (n, 5), Germany (n, 2), Portugal, Denmark, Scotland, Netherlands, Slovenia] reported that 11/16 (69%) of the 16/29 (55%) of laboratories which were equipped with pneumatic tubes no longer used them for transfer of samples (42) (Table 1). However, some laboratories continued to use pneumatics for transfer of fresh samples if the COVID-19 status of the patient was unknown (Table 1).
Full table
Some new workflows and mandatory procedures have been rapidly set up in pathology laboratories to ensure staff biosafety, according to different international recommendation (43). Due to the virulence of SARS-CoV-2 gross macroscopy and dissection of non-fixed surgical specimens and frozen section procedures should require the use of a biological safety cabinet of class II (BSC 2) hood and not a chemical hood (39,40). However, it is not certain that all pathology laboratories are equipped with a dedicated room and BSC 2 for such gross macroscopy and frozen section work, while all have a chemical hood for dissection of surgical specimens. In this regard the organization of pathology laboratories probably varies among institutions where some BSC 2 are used only for handling of fluids and cytological examinations (such as bronchial aspirates, pleural liquid, BAL), and are not installed in rooms for gross macroscopy of fresh surgical specimen. An enquiry performed with the 29 above-mentioned laboratories reported that 121/29 (73%) performed dissections of non-fixed lungs, notably during frozen section procedure under chemical hoods or even on tables equipped with aspiration (Table 1). It should be pointed out that the accreditation of pathology laboratories in Europe requires the ISO 15189 norm and the chapter concerning the hygiene and security of the personal does not give a lot of detail in this domain (44). Interestingly, the control by mandated auditors designated by the organizations issuing ISO 15189 accreditation norm, of the transfer of non-fixed samples into the pathology laboratories and the handling under chemical and/or microbiological hoods is not explicitly stipulated by this norm (44).
High level of precautions to protect the staff working at the macroscopic level while handling non-fixed surgical specimens, cytological samples or liquids (BAL and cell counts, bronchial aspirates and swabs, fine-needle aspirations, pleural and cerebrospinal fluids and blood) must be systematically adopted during this pandemic, whether the COVID-19 status of the patient is known or not. The optimal precautions include wearing an effective protective masque [filtering facepiece (FFP) 2, FFP3 or N95 respiratory type], two pairs of gloves, eye glasses or a protective screen, head ware, shoe covers, disposable laboratory coats and plastic aprons (5,37,39,40,45-47). All personnel must be equipped with this protective ware before entering the room where samples are manipulated and the door kept closed after entering of each personnel. The procedures for evacuation of the rubbish must be known by all laboratory staff and the elimination of the rubbish must be controlled via a well-established one-way circuit into the laboratory. Strict measures of disinfection of the different surfaces of the laboratory must be applied several times during the day (38). The survey made from the 29 laboratories revealed that the practices of the different pathology laboratories varied considerably from a laboratory to another one: 9/29 (31%) and 20/29 (69%) laboratories equipped the staff with FFP2/N95 respiratory and surgical masks, respectively; two pairs of gloves were used in three laboratories only; a plastic apron over a disposable coat were used in two other laboratories only; 19/29 (65%), 8/29 (28%) and 7/29 (24%) used disposable coats, surgical cap and surgical overshoes respectively. All the 29 (100%) laboratories used for eye protection (plastic glasses or safety face shields) (Table 1).
When considering the above-mentioned elements, the question of how to manage non-fixed thoracic samples when the COVID-19 status is not known before surgery or cytological examination, which represents probably the majority of cases until now, can be raised. Recommendations were rapidly issued by different scientific organization at a national level, for example in France, by the society for thoracic and cardio-vascular surgery (48). For this latter society, investigation into the COVID-19 status using reverse transcriptase polymerase chain reaction (RT-PCR) from nasopharyngeal samples should be performed for only febrile patients or for those showing-suggestive-images of COVID-19 infection on computerized tomography (CT) scans (48). So infected asymptomatic patients who are nonetheless contagious are currently not test for the virus before thoracic surgery, at least in certain institutions and countries (49,50). Thus, without knowing it, it is possible that non-fixed tissue and cell samples from patients with COVID-19 are handled in the pathology laboratory. Transport by pneumatic tubes may keep going to operate in several laboratories, in the absence of systematic detection of SARS-CoV-2 infections in patients (38). Consequently, a number of hospitals set up RT-PCR for detection of the virus in cellular nasopharyngeal samples. Among the 29 pathology laboratories consulted only 4/29 (14%) obtained the COVID-19 status of the patients treated in the department of thoracic surgery before receiving the samples (Table 1). Knowledge concerning the degree of contagiousness and of the virulence of SARS-CoV-2 has evolved rapidly, but a number of uncertainties persist (51). So, this virus is certainly more contagious than other coronaviruses including SARS-CoV-1. Thus, asymptomatic patients and/or those at a stage of incubation can transmit the infection several days before becoming immunized and experiencing symptoms such as fever and cough. Moreover, according to some studies, detection of the virus by RT-PCR can give a negative result in a significant percentage of cases (52). Thus, a first negative PCR result can require an additional sample for a new test 48 hours later. In this context, it should be possible to transfer samples from patients who have two negative tests using pneumatic tubes. Samples from positive patients are carried manually from the surgical and clinical departments to the laboratory and gross macroscopic examination of the non-fixed surgical specimens as well as cytological samples and fluids are handled according to the precautions mentioned above. The question as to whom and when to test by RT-PCR can be raised. Ideally, this should be done first during the first visit with the anesthetist and second the day before surgery or endoscopy, thus, ensuring that all patients are not infectious up to the time of hospitalization and surgery. The possibility of testing for specific antibodies in plasma before surgery can also be considered, knowing however that positivity does not mean total absence of viral particles in the respiratory tree and that the level of security is lower than with results of RT-PCR from the respiratory epithelium (53).
SARS-CoV-2 is inactivated after an as yet undetermined time when samples are fixed in formalin (38). The steps of dehydration and tissue embedding in paraffin also completely inactivate the virus. However, the question of how long the virus survives on certain surfaces still persists, particularly on certain plastic. The disinfection of cassettes from formalin-fixed paraffin-embedded (FFPE) tissue with alcohol-based gels (containing 70% ethanol and 0.1% sodium hypochlorite) may also be recommended (38,40).
Since recent years, a number of pathology laboratories perform blood tests for detection of genomic alterations of patients with advanced non-small cell lung carcinomas, in particular for mutations in epidermal growth factor receptor (54). These tests require centrifugation within a few hours of blood sampling of tubes containing an anticoagulant. These tubes can be sent via pneumatic tubes or carried by hand. According to different studies the risk of infection with SARS-CoV-2 from blood samples is very low and is detected in only 1% of samples (10). Nonetheless, the possible presence of SARS-CoV-2 in plasma and in pellets of leucocytes requires the same degree of precaution as mentioned above, in particular when the patient is known to carry the virus in the nasopharynx (55).
The set up and adoption of the practices described above necessitate new rules for the procedures employed in the pathology laboratory and for the work of the pathologists and technicians. This has probably a more or less important impact on the delay of transmission of the results and on the budget of the laboratory.
Management of tissue and liquid samples in biobanks
The transmission to the biobank of tissue, cytological and liquid samples from patients with lung cancer and then their management occur most often after a quality control of these samples in a laboratory hospital aiming to provide care for these patients. Thus, samples initially obtained for diagnostic purposes are requalified to translational research purpose. The mastering of collections within biobanks probably differs depending on whether the hospital laboratory and the biobank are on the same site (same floor or same building) or on several sites and if one or different staff members manage the pathology laboratory and/or the biobank. The traceability, quality control and the information associated with the sample as well as the rapid transfer from the care zone to the biobank is probably facilitated by close contact of the two structures and if the responsibility for the management is shared by the same team (56). The management of the sample transfer and of the different procedures is optimal if the same team is aware of the norms for quality that exist in both the pathology laboratory and the biobank. This may become even more important in the context of the COVID-19 pandemic since the traceability of the associated data of the sample is particularly valuable and mandatory.
Three situations can be described:
- The samples come from SARS-CoV-2 infected patients (RT-PCR positive). Disregarding the type of sample (fresh or frozen tissues, cells, biological fluids such as pleural or cerebrospinal fluids, blood and blood derived products FFPE tissues, feces, etc.) they are all considered to be potentially infectious. Information must be transferred to the hospital laboratory and reception of this information by members of the biobank, including the head of the biobank, must be acknowledged. Before transfer of any sample to the biobank all containers must be disinfected (tubes, plastic cassettes) with the appropriate solutions (alcohol-based gels and/or bleach). All samples must be stored in a zone separate to those for storage of samples before the COVID-19 pandemic.
- The samples from patients tested negative by RT-PCR the day before their transfer to the pathology laboratory. Ideally the containers are disinfected as mentioned above (since these samples come from a hospital laboratory handling potentially infectious material from COVID-19 positive patients) but can be stored in the biobank with samples collected before the COVID-19 pandemic. However, “zero” risk does not exist even with two nasopharyngeal cell samples RT-PCR test negative. Thus, in theory all the samples could be considered as potentially infectious (17).
- The samples come from patients for whom the COVID-19 status is not known because no RT-PCR test was performed. In theory, all the samples must be considered as potentially infected by the virus. The risk is particularly high for fresh and frozen tissues, as well as for frozen fluids that are transferred to the laboratory hospital. If non-frozen liquid samples are transferred directly from a hospital laboratory to a biobank they must be systematically aliquoted under a BSC 2 by personnel appropriately protected with the afore mentioned clothing and procedure. The handling and storage of the samples must follow the same circuit as that mentioned in the above section number 1. Ideally, the procedures for storage may be modified if serological tests are performed retrospectively on patients to determine if they were infected or not with SARS-CoV-2. However, it is known that some seropositive patients can still be infectious and thus their samples too.
The COVID-19 pandemic and the precautions mentioned above require transmission of even more specific information that is essential to the staff working or going to work in biobanks. This different information can be transmitted in particular through different specialized education and training programs (57,58).
Requests by the end users
Research scientists of academia domains and of the biotechnology and pharmaceutical industries need many tissue, cell or liquid samples for translational research. Thanks to these samples from lung cancer patients and associated high quality clinical data research into thoracic oncology has evolved considerably during the last few years, leading to the discovery of diagnostic, prognostic and theranostic biomarkers. The use of biological samples by scientists is associated with legal and ethical regulations, the establishment of a material transfer agreement and by requirements presented by different partners (from the hospital executive, the biobanker, and the scientist) (59,60). These requirements take into account the guidelines adopted by the respective countries and the regulations for importation and exportation of human samples. In all cases traceability of the samples must be assured by providing information concerning the origin of biospecimens, in respecting the laws of the country from which the samples are obtained and indicating their final use (61). These different points are presently part of the work of the biobanks and are essential to certification according to the ISO-9001:2015 and S96-900 norms or to accreditation according to the ISO 20387:2018 norm (62-64). The COVID-19 pandemic may result in an immediate decrease in the collection and use of biological samples for research, in particular into thoracic oncology (65). It is essential to respect all the procedures mentioned above: The biobank delivering samples to scientists could mention the COVID-19 status of the patient but this point must then appear on the informed consent signed by the patient and be mention as soon as possible in the near future. Thus, distinction between RT-PCR testing for the virus at one or two times separated by 48 hours, including the day before the sampling, or serological testing must be done. Results of RT-PCR or serological testing may be requested by the end users in the near future before using samples from lung cancer patients. This seems mandatory for fresh or frozen tumor lung samples, but may also be applied to tissue-derived products (such as nucleic acids), fluids or even for tissues and FFPE cells. Scientists may also request that the tubes and paraffin plastic cassettes be disinfected prior to transfer and ensure that the transport of the packages respects safety requirements. Thus, the budget set aside for the use of biological samples for research purposes may increase (66). Transport of the samples by the biobank must follow guidelines already established for potentially infectious human samples. Transport may be the responsibility of the biobank or under the responsibility of the carrier who must thus have proof of expertise in this domain.
Handling of non-formaldehyde fixed samples by the end-users must be done respecting the above-mentioned precautions, in particular under a PSM2 hood and by appropriately equipped and protected staff, notably with FFP2 or FFP3 masks. The culture of cells from tumor samples leads to a number of important questions concerning security when the COVID-19 status of the patient is not known. However, in this context all samples must be considered as potentially infectious for the SARS-CoV-2, which requires manipulation in a BSL3 environment (29,37-40).
Conclusions
The COVID-19 pandemic has had and will have further consequences on translational research in thoracic oncology. In fact, the number of both frozen and fixed samples collected since the beginning of the pandemic in hospital laboratories and subsequently managed by biobanks has probably decreased considerably, even if there are no global estimates to date. For example, the number of patients with lung cancer who were operated on and from whom tissue samples (FFPE) were collected in the biobank of the Nice University Hospital (France) between January 1 and April 15 in 2019 was 155 samples from 198 operated patients (78%) vs. 68 for 165 operated patients (41%) for the same period in 2020 (personal communication). The decrease of collected samples may be linked to the urgent reorganization of patient care and of the pathology laboratories of the hospitals, but also to the potential slowing of research into thoracic oncology, to the need to reorientate the work of staff providing patient care, to the influx of patients with COVID-19 and to the adoption of very strict guidelines for hygiene and safety for the handling of lung cancer samples in both hospital laboratories and biobanks. As soon as the COVID-19 pandemic was declared it was urgent to adopt in a retrospective and prospective way some new recommendations for the collection, storage and transfer of tumor samples, in particular because a number of uncertainties still exist concerning the SARS-CoV-2 and its virulence (51). In this context establishment of best practices to support biological safety of the personal working in different laboratories is mandatory (Table 2). Collect samples such as nasopharyngeal swabs, BAL and blood from infected patients in biobanks from different countries is certainly a critical point for the development of near research programs for better knowledge of the SARS-CoV2 pathophysiology (67). However, a major issue is also to maintain enough high-quality biological samples and associated clinical data to continue to develop translational research projects in thoracic oncology since the number of accessible samples for end-users may rapidly decrease in the near future.

Full table
Acknowledgments
The author thanks Massimo Barberis MD, Fabrizio Bianchi MD, Reinhard Büttner MD, Fiorella Calabrese MD, Lina Carvahlo MD, Paolo Graziano, Birgit, Guldhammer Skov MD, Keith Kerr MD, Izidor Kern MD, Fernando Lopez-Rios MD, Giuseppe Pelosi MD, Teodora Radonic MD, Michael Roehrl MD, Albrecht Stenzinger MD, Erik Thunnissen MD, Giancarlo Troncone MD, the French pathologists of the PATTERN group (Julien Adam MD, Martine Antoine MD, Cécile Badoual MD, Hugues Begueret MD, Frédéric Bibeau MD, Aurélie Cazes MD, Jean-François Côté MD, Diane Damotte MD, Fabien Forest MD, Stéphane Garcia MD, Véronique Hofman MD, Elodie Long, MD, Sandra Lassalle MD, Marius Ilie MD, Sylvie Lantuejoul MD, Jean-Christophe Sabourin MD, Lucie Tixier MD), the Cancéropôle PACA, the Ligue Départementale 06 de Lutte contre le Cancer, and the Conseil Départemental 06.
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-594). PH serves as an unpaid editorial board member of Translational Lung Cancer Research from Feb 2019 to Feb 2021.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335-7. [Crossref] [PubMed]
- Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020;21:e181. [Crossref] [PubMed]
- Xia Y, Jin R, Zhao J, et al. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020;21:e180. [Crossref] [PubMed]
- Calabrò L, Peters S, Soria JC, et al. Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir Med 2020;8:542-4. [Crossref] [PubMed]
- Cook TM. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic - a narrative review. Anaesthesia. 2020;75:920-7. [Crossref] [PubMed]
- Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020;104:246-51. [Crossref] [PubMed]
- Geller C, Varbanov M, Duval RE. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 2012;4:3044-68. [Crossref] [PubMed]
- Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol 2020;153:420-1. [Crossref] [PubMed]
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564-7. [Crossref] [PubMed]
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323:1843-4. [PubMed]
- Pambuccian SE. The COVID-19 pandemic: implications for the cytology laboratory. J Am Soc Cytopathol 2020;9:202-11. [Crossref] [PubMed]
- Vigliar E, Iaccarino A, Bruzzese D, et al. Cytology in the time of coronavirus disease (covid-19): an Italian perspective. J Clin Pathol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Baig AM, Khaleeq A, Ali U, Syeda H, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995-8. [Crossref] [PubMed]
- Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev 2020;34:75-80. [Crossref] [PubMed]
- Chu H, Chan JF, Wang Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv 2020. doi:. [Crossref]
- Lei Z, Cao H, Jie Y, et al. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis 2020. [Epub ahead of print].
- Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69:997-1001. [Crossref] [PubMed]
- Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020. [Epub ahead of print]. [PubMed]
- Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465-9. [Crossref] [PubMed]
- Xu J, Li Y, Gan F, et al. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456-74. [Crossref] [PubMed]
- Lukassen S, Chua RL, Trefzer T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 2020;39:e105114. [Crossref] [PubMed]
- Rivellese F, Prediletto E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev 2020;19:102536. [Crossref] [PubMed]
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Malapelle U, De Luca C, Iaccarino A, et al. Predictive molecular pathology in the time of COVID-19. J Clin Pathol 2020. [Epub ahead of print].
- Minucci A, Scambia G, Santonocito C, et al. BRCA testing in a genomic diagnostics referral center during the COVID-19 pandemic. Mol Biol Rep 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Homer LC, Alderman TS, Blair HA, et al. Guidelines for biosafety training programs for workers assigned to BSL-3 research laboratories. Biosecur Bioterror 2013;11:10-9. [Crossref] [PubMed]
- Li L, Gu J, Shi X, et al. Biosafety level 3 laboratory for autopsies of patients with severe acute respiratory syndrome: principles, practices, and prospects. Clin Infect Dis 2005;41:815-21. [Crossref] [PubMed]
- Nolte KB, Taylor DG, Richmond JY. Biosafety considerations for autopsy. Am J Forensic Med Pathol 2002;23:107-22. [Crossref] [PubMed]
- Ta L, Gosa L, Nathanson DA. Biosafety and biohazards: understanding biosafety levels and meeting safety requirements of a biobank. Methods Mol Biol 2019;1897:213-25.
- Burnett LC, Lunn G, Coico R. Biosafety: guidelines for working with pathogenic and infectious microorganisms. Curr Protoc Microbiol 2009;Chapter 1:Unit 1A.1.
- Howanitz JH, Howanitz PJ. Laboratory results. Timeliness as a quality attribute and strategy. Am J Clin Pathol 2001;116:311-5. [Crossref] [PubMed]
- Manor PG. Turnaround times in the laboratory: a review of the literature. Clin Lab Sci 1999;12:85-9. [PubMed]
- Stadler S. Pneumatic tube system: optimum approach to stat specimen transport. MLO Med Lab Obs 1989;21:55-7, 60-2. [PubMed]
- Centers for Disease Control and Prevention (CDC). Interim laboratory biosafety guidelines for handling and processing specimens associated with coronavirus disease 2019 (COVID-19). Available online: (Accessed March 7, 2020).https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html
- Henwood AF. Coronavirus disinfection in histopathology. J Histotechnol 2020;43:102-4. [Crossref] [PubMed]
- World Health Organization. Laboratory biosafety guidance related to the novel coronavirus (2019-nCoV): interim guidance. Available online: (Accessed March 7, 2020).https://www.who.int/docs/default-source/coronaviruse/laboratory-biosafety-novel-coronavirus-version-1-1.pdf?sfvrsn=912a9847_2
- Wu W, Lei H. A Practical Handbook for Infection Control in Makeshift (Fangcang) Hospitals: Experience from Coronavirus Disease 2019 (COVID-19) Pandemic. Shanghai: Shanghai Scientific & Technical Publishers, 2020.
- Barkham TM. Laboratory safety aspects of SARS at biosafety level 2. Ann Acad Med Singapore 2004;33:252-6. [PubMed]
- Lantuejoul S, Adam J, Girard N, et al. PD-L1 testing in non-small cell lung carcinoma: Guidelines from the PATTERN group of thoracic pathologists. Fondation Synergie Lyon Cancer. Ann Pathol 2018;38:110-25. [Crossref] [PubMed]
- Gosney JR, Hofman P, Troncone G, et al. Cellular pathology in the COVID-19 era: a European perspective on maintaining quality and safety. J Clin Pathol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Long-Mira E, Washetine K, Hofman P. Sense and nonsense in the process of accreditation of a pathology laboratory. Virchows Arch 2016;468:43-9. [Crossref] [PubMed]
- Iwen PC, Stiles KL, Pentella MA. Safety Considerations in the laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J Clin Pathol 2020;153:567-70. [Crossref] [PubMed]
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Institute of Biomedical Science. COVID19-recommendations for laboratory work. Available online: (Accessed March 29, 2020).https://www.ibms.org/resources/news/covid-19-recommendations-for-laboratory-work/
- COVID19-recommandations de la Société Française de Chirurgie Thoracique et Cardio-Vasculaire Cardiaque (SFCTCV). Available online: (Accessed March 23, 2020).https://www.sfctcv.org/
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020;323:1406-7. [Crossref] [PubMed]
- Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020;382:970-1. [Crossref] [PubMed]
- Weston S, Frieman MB. COVID-19: knowns, unknowns, and questions. mSphere 2020;5:e00203-20. [Crossref] [PubMed]
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin Chem Lab Med 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Vashist SK. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics (Basel) 2020;10:202. [Crossref] [PubMed]
- Heeke S, Benzaquen J, Hofman V, et al. Critical assessment in routine clinical practice of liquid biopsy for EGFR status testing in non-small-cell lung cancer: a single-laboratory experience (LPCE, Nice, France). Clin Lung Cancer 2020;21:56-65.e8. [Crossref] [PubMed]
- Wang X, Xu W, Hu G, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol 2020. [Epub ahead of print]. [Crossref]
- Washetine K, Heeke S, Bonnetaud C, et al. Establishing a dedicated lung cancer biobank at the University Center Hospital of Nice (France). Why and how? Cancers (Basel) 2018;10:220. [Crossref] [PubMed]
- Gormally E, Hardy I, Caboux E, et al. Training the next generation of biobankers: a two-year master's course in the management of biobanks. Biopreserv Biobank 2017;15:438-50. [Crossref] [PubMed]
- Université Côte d'Azur. MSc Biobanks and Complex Data Management. Available online: http://univ-cotedazur.fr/en/education/informations-utiles/les-informations-utiles/biobanks-complex-data#.XpnieGSYSJA
- Hofman P, Bréchot C, Zatloukal K, et al. Public-private relationships in biobanking: a still underestimated key component of open innovation. Virchows Arch 2014;464:3-9. [Crossref] [PubMed]
- Simeon-Dubach D, Roehrl MH, Hofman P, et al. Enhancing cooperation between academic biobanks and biomedical industry: better mutual understanding and new collaborative models are needed. Biopreserv Biobank 2020;18:144-9. [Crossref] [PubMed]
- Cooreman A, Bravo E, van Gool AJ, et al. Point of view: traceability and transparency should be mandatory for all human biospecimens. Appl Clin Trials 2017. Available online: http://www.appliedclinicaltrialsonline.com/point-view-traceability-and-transparency-should-be-mandatory-all-human-biospecimens
- International Organization for Standardization. ISO 9001:2015 Quality Management System—Requirements.
- AFNOR. NF S96-900 Biological Resources Centers quality—BRC Quality Management System, human and microbial biological resource quality.
- International Organization for Standardization. ISO 20387:2018 Biotechnology—Biobanking—General requirements for biobanking.
- Hofman P, Puchois P, Brest P, et al. Possible consequences of the COVID-19 pandemic on the use of biospecimens from cancer biobanks for research in academia and bioindustry. Nat Med 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Clément B, Yuille M, Zaltoukal K, et al. EU-US Expert Group on cost recovery in biobanks. Public biobanks: calculation and recovery of costs. Sci Transl Med 2014;6:261fs45. [Crossref] [PubMed]
- Vaught J. Biobanking during the COVID-19 pandemic. Biopreserv Biobank 2020. [Epub ahead of print]. [Crossref] [PubMed]


