
The CT appearance pattern of radiation-induced lung injury and tumor recurrence after stereotactic body radiation therapy in early stage non-small cell lung cancer
Introduction
In 2018, there were approximately 4.3 million new cancer cases and 2.9 million cancer deaths in China according to cancer statistics, and the total number of cancer cases is expected to reach around 4.51 million with 3.04 million cancer deaths by 2020 (1). Lung cancer is the most commonly diagnosed cancers (21.9% of total cases) and also causes the most cancer-related deaths (26.4% of total cases) in Chinese males. For Chinese females, lung cancer has the second highest incidence (13.3% of total cases) and the highest mortality (20.3% of total cases) (1).
Surgery and stereotactic body radiation therapy (SBRT) are considered as the main treatment options for non-small cell lung cancer (NSCLC), and SBRT in stage I NSCLC has been reported to have a 3-year local control of 92% to 98%, and a 3-year overall survival of 90% (2-4). SBRT has higher biologically effective doses (BED) irradiated during a short period, realizing the higher radiative dose of the tumor, meanwhile minimizing the exposure of the organ at risk, and achieve successful local control of lung cancer. Thus, SBRT is thought to be an optimal treatment modality for patients who have inoperable disease or who refuse operation by virtue of its good local control and low incidence of severe toxicities, with several studies finding SBRT to be an equivalent treatment option for early-stage NSCLC (2,3). This approach can also reduce overall treatment time from several weeks of conventional radiotherapy to a few days, which offering a convenience to the patients. Radiation-induced lung injury (RILI) include radiation-induced pneumonitis and lung fibrosis, and radiation pneumonitis is a predominant complication after radiotherapy while the incidence of severe radiation pneumonitis after SBRT is low (0–29%); however, more than half of patients who are treated with SBRT develop radiographic patterns of RILI (5,6). It is widely known that the CT pattern of early and late benign fibrosis is commonly observed after SBRT for lung cancer, and response evaluation criteria in solid tumor (RECIST)-defined tumor recurrence depends on the variation of lesions size. However, as some radiation-induced modifications arise from inflammation and fibrosis, it usually takes a period of time to evaluate treatment response, which limits the application of RECIST (7,8). Some changes on CT imaging can be associated with a higher risk for tumor recurrence, and these are known as high-risk features (HRFs). Huang et al. summarized the CT radiographic changes on acute and late lung injury after SBRT and the HRF of local recurrences, found the following characteristic patterns: an enlarging opacity of preliminary focus, a bulging margin of opacity, disappearance (including partial disappearance) of the linear edge and air bronchograms, and opacity enlarging in a craniocaudal direction and they noted that the sensitivity and specificity of identification on tumor recurrence reached up to 90% with the simultaneous presence of 2 or more HRFs (9-11).
Herein, we discuss our investigation of the CT appearance pattern of RILI and recurrence after SBRT in patients with early stage NSCLC in order to better identify RILI and local disease failure.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-609).
Methods
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained at institutional review board of Shanghai Pulmonary Hospital for this retrospective study (approval ID: K20-174Y). Informed consent was waived.
Patients and radiotherapy
We selected 60 patients with early stage NSCLC who received SBRT treatment in Shanghai Pulmonary Hospital and Cancer Hospital affiliated to the University of the Chinese Academy of sciences from February 2012 to June 2018, and retrospectively analyzed their clinical data. The study cohort comprised 44 males and 16 females, and the median age was 76 (range, 52–88) years. The specific information of enrolled patients is shown in Table 1. The median prescription dose was 50 Gy/5 fraction (range, 40–70 Gy/4–10 fraction). All patients had a biologically effective dose (BED10) of ≥100 Gy, and the total dose covered 95% of the planning target volume (PTV).
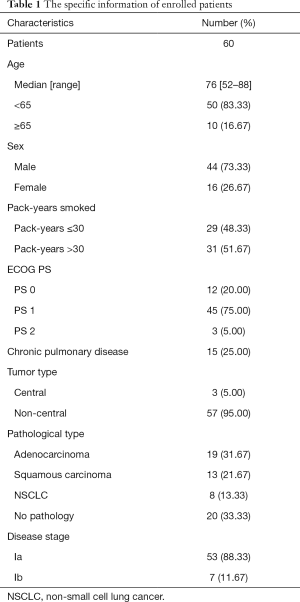
Full table
Follow-up
All patients had undergone serial CT scanning before SBRT and every 3 months for the first half year, every 6 months for the next half year (at 3, 6, 12, 18, and 24 months post-treatment), and then annually after completion of SBRT. The frequency of CT scan increased when relapse was highly suspected, and the relapse was verified with 18F-FDG (positron emission tomography) PET/CT or biopsy. In the present study, all the enrolled patients underwent 18F-FDG PET/CT scanning. A standard uptake value (SUV) greater than 5 or an initial value (as measure by initial 18F-FDG PET/CT scanning) was diagnosed as tumor relapse according to the literature (10,12). In the present study, 7 patients were diagnosed with relapse, and of these patients, 1 patient had the relapse verified by pulmonary fine needle puncture pathology, while 6 patients failed biopsy because of cardiopulmonary dysfunction or old age. In this instance, the diagnostic criteria of relapse were opacity increase on CT scanning after SBRT, SUV measurements by 18F-FDG PET/CT revealed as positive and higher than those before treatment. An experienced radiation oncologist and radiologist who were blinded reviewed all CT images and identified acute or late RILI and HRF.
Statistical analysis
Statistical analysis was conducted using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). The chi-squared was used to assess the consistency between the two observers and to compare radiographic changes between the recurrence and non-recurrence groups. P value <0.05 was considered statistically significant.
Results
Patients data
The median follow-up was 36.7 months (3–70 months). There were 7 patients with recurrence after treatment in 60 patients, and 1 patient with RECIST-indicated progressive disease. 1- and 3-year overall survival were 95.2%, 86.3%, and 1- and 3-year progression-free survival were 85.9%, 69.4%. There were 55 patients with radiation injury, and the incidence of RILI was 91.67%. The median time for the occurrence of RILI was 4 months (1–12 months), and the median time for the endpoint of the RILI progression was 12 months (4–34 months).
The acute and late RILI
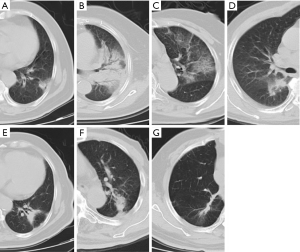
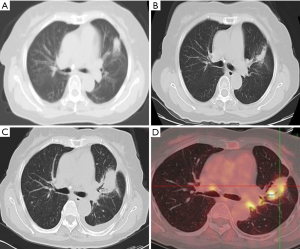
In the follow-up of 7 patients with recurrence, 1 case had no RILI, 2 cases had diffuse consolidation, 4 cases had patchy consolidation and GGO, 3 cases had mass-like pattern, and 3 cases had modified conventional pattern. The characteristics CT changes of patients with recurrence are shown in Table 2. The CT features of acute and late RILI are shown in Figure 1.
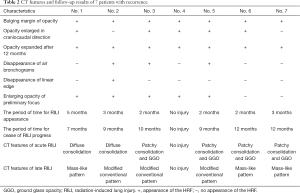
Full table
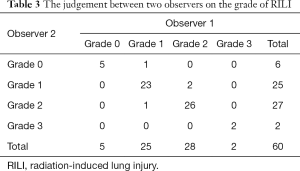
There were differences between the 2 observers in judging the grade of RILI, as shown in Table 3. The Chi-squared test was used to analyze the consistency between the 2 observers on the grade of RILI, and the Kappa coefficient was 0.89, indicating that the judgment of RILI grade by the 2 observers had good consistency. The grade 1 and grade 2 RILI were considered the primary event.
Full table
The CT characteristics of acute and late RILI are shown in Tables 4 and 5. The Chi-squared test was used to analyze the consistency between the 2 observers on CT characteristics of acute and late RILI, and the Kappa coefficients were 0.706 and 0.72. These showed that the judgement of 2 observers on CT characteristics of acute and late RILI had good consistency. Observer 1 found the patchy consolidation and GGO to be the main CT feature on acute RILI, while observer 2 found patchy GGO to be the main feature. The modified conventional pattern was the main CT feature on late RILI.
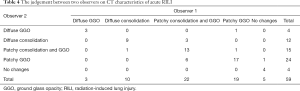
Full table

Full table
High-risk features
The enlarging opacity of preliminary focus (29, 48.33%) and bulging margin of opacity (19, 31.67%) were the primary HRFs among the CT images of 60 patients. The bulging margin of opacity and the opacity expanding after 12 months had the highest sensitivity (both of 100%), while disappearance of air bronchograms had the highest specificity (100%). There was no statistically significant difference between the disappearance of linear edge and the enlarging opacity of preliminary focus. The odds ratio (OR) value of the disappearance of linear edge was less than 1, and the OR value of the opacity expanding after 12 months reached up to 216.4. The incidence of high-risk features was detailed in Table 6.
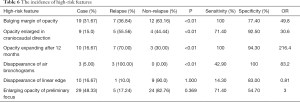
Full table
The accumulated sensitivity and specificity of HRFs are shown in Table 7. Patients with only 1 HRF showed high sensitivity (100%) and low specificity (52.80%), and the specificity increased with the number of HRFS while the sensitivity decreased. The CT images with diagnosis, follow-up, and relapse of 1 patient are shown in Figure 2. The tumor relapse was confirmed by PET/CT on the 53rd month, and we found 2 HRFs on follow-up CT image, including the opacity expanding after 12 months and the bulging margin of opacity.
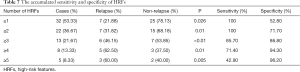
Full table
Discussion
We found 1- and 3-year overall survival in the 60 patients were 95.2% and 86.3%. The therapeutic effectiveness SBRT was desirable and the incidence of RILI was 91.67%. The 2 observers had a good agreement on the grade of RILI, and the CT pattern of acute and late RILI. We found that the incidence of severe RILI after SBRT was extremely low, and grade 1 and grade 2 RILI were the primary pulmonary toxicities. Patchy consolidation and GGO was the most common CT pattern on acute RILI, and the modified conventional pattern was mainly found with late RILI. The specificity of relapse tumor increased with the number of HRFS while the sensitivity decreased, which was important for the diagnosis of tumor recurrence.
One retrospectively study analyzed the SBRT outcomes of 88 operable patients with early-stage NSCLC. For toxicities, grades 0, 1, 2 and 3 radiation pneumonitis occurred in 37.5%, 47.7%, 13.6%, and 1.1% of patients, respectively, and no grade 4 or 5 radiation pneumonitis occurred (2). In the present study, observer 1 found that grades 0, 1, 2, and 3 RILI were 10%, 41.7%, 45%, and 3.3%, respectively, while observer 2 found that grades 0, 1, 2, and 3 RILI were 8.3%, 41.7%, 46.7%, and 3.3%, respectively. Thus, the incidence rate of severe RILI after SBRT is low, and mild RILI after SBRT warrants greater attention.
Trovo et al. (13) enrolled 68 patients who accepted SBRT and found some patients had acute RILI at 6 weeks after SBRT, most patients had no findings (37, 54.4%), and 11 patients had patchy GGO. In their follow-up, patchy consolidation and GGO was the primary pattern 2–6 months after SBRT, and the modified conventional pattern was the main pattern in late RILI 7–18 months after treatment. The findings are consistent with those of the present study. Hayashi et al. (8) investigated 81 NSCLC patients after SBRT, and 6 patients had tumor relapse, including 5 case of mass-like opacity pattern and 1 case of modified conventional pattern. The present study had 3 cases of mass-like pattern, 3 cases of modified conventional pattern, and 1 case of no injury. That is to say, the mass-like and modified conventional pattern were the main CT patterns of late RILI. We also found that among the 7 patients with relapse of acute RILI, there were 2 cases of diffuse consolidation, 4 cases of patchy consolidation and GGO, and 1 case of no injury. The radiological changes developed in most patients after SBRT, but several researchers have reported slightly different results concerning the main CT pattern. These differences may depend on which delivery technique was chosen, as the frequency and timing of the radiological changes can vary according to the delivery technique (14). Ronden et al. (11) described the typical radiological changes in patients who had undergone SBRT with an older fixed-beam delivery approach, and the most frequent acute radiological changes were diffuse consolidation and patchy consolidation. However, many institutions currently use a modern delivery technique, for which late radiological changes are more common, with the main CT pattern being a modified conventional pattern (62%) that is difficult to distinguish from local recurrence (11,14).
Huang et al. retrospectively analyzed a total of 88 patients who were treated with SBRT using volumetric-modulated arc therapy, and they found that the most frequently observed HRFs in non-recurrence patients were enlarging opacity (64.8%) and enlarging opacity after 12 months (50.0%) (9). The present study indicated that the enlarging opacity of preliminary focus (48.33%) also had the highest incidence, while there were 7 patients who were diagnosed with tumor recurrence. We also found that opacity expanding after 12 months had the highest OR (216.4) which was the important indicator for recurrence diagnosis. We can thus definitively conclude that enlarging opacity or opacity expanding after 12 months alone cannot be depended on to determine tumor recurrence. In our study, we also found that the specificity increased while the sensitivity decreased as the number of HRFs increased. Therefore, regular follow-up and attention to high-risk HRFs are crucial.
The radiomics approach may detect early changes associated with local recurrence by extracting quantitative features, and these features are not typically considered by physicians (9). Moran et al. (6) found radiomics features significantly correlated with radiation oncologist-scored post-SBRT lung injury and showed a significant dose-response relationship. Thus, radiomics may help us have a better understanding of pulmonary toxicity and tumor relapse and may facilitate the earlier intervention of local recurrences after SABR (6,9). In the future, our group will also use the radiomics method to investigate pulmonary toxicity after SBRT.
In summary, regular follow-up and attention to high-risk HRFs are vital in better identifying RILI and local disease failure.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-609
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-609
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-609). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained at institutional review board of Shanghai Pulmonary Hospital for this retrospective study (approval ID: K20-174Y). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer 2017;109:62-7. [Crossref] [PubMed]
- Bang A, Bezjak A. Stereotactic body radiotherapy for centrally located stage I non-small cell lung cancer. Transl Lung Cancer Res 2019;8:58-69. [Crossref] [PubMed]
- Perspectives on stereotactic body radiotherapy for early-stage non-small cell lung cancer: a maturing treatment modality. J Thorac Dis 2018;10:1207-10. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017;123:3031-9. [Crossref] [PubMed]
- Moran A, Daly ME, Yip SSF, et al. Radiomics-based Assessment of Radiation-induced Lung Injury After Stereotactic Body Radiotherapy. Clin Lung Cancer 2017;18:e425-e431. [Crossref] [PubMed]
- Tyran M, Charrier N, Darreon J, et al. Early PET-CT After Stereotactic Radiotherapy for Stage 1 Non-small Cell Lung Carcinoma Is Predictive of Local Control. In Vivo 2018;32:121-4. [PubMed]
- Hayashi S, Tanaka H, Hoshi H. Imaging characteristics of local recurrences after stereotactic body radiation therapy for stage I non-small cell lung cancer: Evaluation of mass-like fibrosis. Thorac Cancer 2015;6:186-93. [Crossref] [PubMed]
- Huang K, Senthi S, Palma DA, et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol 2013;109:51-7. [Crossref] [PubMed]
- Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol 2012;102:335-42. [Crossref] [PubMed]
- Ronden MI, Palma D, Slotman BJ, et al. Brief Report on Radiological Changes following Stereotactic Ablative Radiotherapy (SABR) for Early-Stage Lung Tumors: A Pictorial Essay. J Thorac Oncol 2018;13:855-62. [Crossref] [PubMed]
- Huang K, Palma DA. IASLC Advanced Radiation Technology Committee. Follow-up of patients after stereotactic radiation for lung cancer: a primer for the nonradiation oncologist. J Thorac Oncol 2015;10:412-9. [Crossref] [PubMed]
- Trovo M, Linda A, El Naqa I, et al. Early and late lung radiographic injury following stereotactic body radiation therapy (SBRT). Lung Cancer 2010;69:77-85. [Crossref] [PubMed]
- Senthi S, Dahele M, van de Ven PM, et al. Late radiologic changes after stereotactic ablative radiotherapy for early stage lung cancer: a comparison of fixed-beam versus arc delivery techniques. Radiother Oncol 2013;109:77-81. [Crossref] [PubMed]


