
Integrative approach to cytologic and molecular diagnosis of malignant pleural mesothelioma
Background
The global incidence and mortality from malignant mesothelioma (MM) varies greatly over the world (1,2). The geographic distribution pattern of MM is linked to the use of various mineral fibers with carcinogenic properties. Clinical symptoms occur late in the course of the disease and the need to demonstrate tumor invasion in the surrounding tissue (3) further delay the diagnosis. Early diagnosis would provide extended symptom control, better possibility for treatment (4) and prolonged patient survival (5).
Unilateral accumulation of pleural effusion is one of the earliest clinical manifestations of MM that occurs in up to 90% of the patients. Therapeutic thoracocenthesis is necessary to remove the fluid and to relieve patients’ symptoms. This effusion is easily accessible and offers early and minimally invasive diagnosis by combining cytology with immunologic, molecular- and biomarker analyses. Typically, the fluid is rich in malignant cells and cell groups, but incipient stages of the disease may be difficult to recognize as the malignant cells can be masked by presence of inflammatory or reactive mesothelial cells. Recurrent, hemorrhagic and cell rich effusion should always be suspicious for MM and adequately prepared and analyzed to provide necessary information for subsequent therapy.
The molecular landscape of MM
Molecular alterations involved in the development of MM are accumulated over several decades, finally leading to malignant transformation of the mesothelial cells. The typical molecular landscape of MM consists of multiple chromosomal losses leading to loss or inactivation of several tumor suppressor genes (6-8). Particularly, chromosomes 3p, 9p and 22q, harboring tumor suppressor genes, are critically involved in the pathogenesis of MM, leading to loss of p16INK4A-p14ARF (CDKN2A) located at 9p21, neurofibromatosis type 2 (NF2) at 22q12 and BRCA1-associated protein 1 (BAP-1) at 3p21.31-p21.2.
Homozygous deletion of CDKN2A or loss of BAP-1 (9,10), are early findings during development of MM. A considerable long term risk to develop invasive MM exists in patients with recurrent pleural effusions harboring BAP1 loss which also seem to be an early event, present several years prior to the finding of an invasive MM. Homozygous deletion of CDKN2A is frequent in MM and apart from FISH analysis of the 9p21 region it can be reliably shown by immunohistochemistry using MTAP as surrogate marker (11). Loss of BAP-1 or 9p21 homozygous deletion results in morphological alteration that reflect these genetic aberration characteristic for MM (12).
The molecular and epigenetic profiles of MM (13-15) differ largely from metastatic adenocarcinoma (14,16,17) and offers a plethora of mutually exclusive molecular markers to differentiate these two conditions as there are little or no overlap between them. Only few MM-related alterations are so far potentially actionable (18). In MM, no single driver mutation has been identified so far, but each patient has a unique setting of alterations, motivating an individualized choice of treatment. Detailed molecular characterization allows stratification of patients in aggressive or indolent subgroups: high score of epithelial to mesenchymal transition (EMT) markers, LATS2 mutation, high AURKA, homozygous deletion of CDKN2A and low mesothelin characterizes the aggressive tumor whereas low copy number alterations, high DNA methylation and high BAP-1 alterations are characteristic of an indolent tumor (19).
Effusion cytology enables early diagnosis: possibilities and challenges
One of the earliest manifestations of MM is a recurrent accumulation of hemorrhagic effusions. To reach a conclusive diagnosis the cytopathologist must be aware of the spectrum of cellular and molecular alterations and recognize the malignant cells present in these early effusions.
The clinical outcome of patients depends largely on the phenotype of the tumor. In pleural effusions, the sarcomatoid tumor components are not exfoliated. Cytomorphology together with adjuvant diagnostic analysis will make the accurate cytologic diagnosis possible in most epithelioid and mixed type MMs, the main diagnostic challenges being the differentiation from benign/reactive mesothelium and from metastatic adenocarcinoma. The positive predictive value of such a cytological diagnosis is as high as for histology (20), and therefore initiation of treatment shouldn’t be delayed. In many countries, however, clinical practice still requires histology and demonstration of invasion of surrounding tissue. This will delay the diagnosis and thereby the initiation of therapy frequently with as much as 6–9 months.
Cytomorphological features of reactive mesothelial hyperplasia versus MM in effusion
In effusions, the exfoliated benign mesothelial cells usually dissociate and form loosely coherent cell conglomerates organized in regular monolayers admixed with inflammatory cells (Figure 1). In some cases the atypia of reactive mesothelial proliferations can be rather pronounced with three dimensional papillary cell groups, posing a challenge to distinguish them from a true malignant process. Inconclusive samples should be tested further in order to exclude malignancy and prove absence or presence of molecular alterations characteristic for MM. Highly cellular effusions with abundant three-dimensional cell groups should always raise the suspicion of a malignant process and should be verified by immunological and/or molecular markers to reach a conclusive diagnosis.
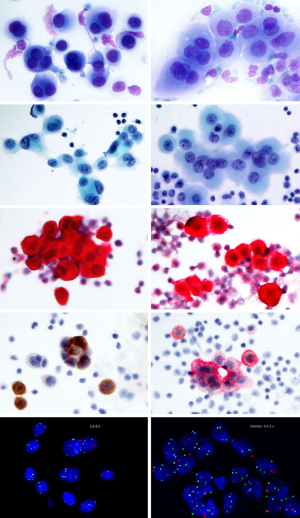
The diagnostic criteria of a malignant effusion include the presence of numerous papillary cell groups of varying size combined with common cytomorphological criteria, such as pleomorphic cells with hyperchromatic, enlarged and or/multiple nuclei, high nuclear/cytoplasmic ratio, presence of macronucleoli, distinct and irregular nuclear contour and variability in structure of the nuclear chromatin (21-24). The majority of samples display clearly malignant cytomorphological characteristics, whereas some cases might be difficult to distinguish from reactive mesothelial hyperplasia. Paucicellular effusions may be challenging as the admixture of inflammatory cells and blood might mask the malignant cells. In these cases, hemolysis and adjuvant methods are required.
The morphological, molecular and immunophenotypic features of MM are well defined and they are concordant in cytology and histology (3,25-28) (Figure 2).
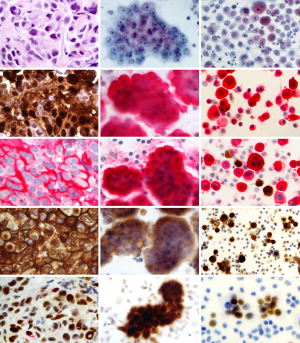
Cytomorphologic findings include protrusions through the cell membrane (“blebbing”); presence of organophilic squamoid cells, fine vacuolization of the perinuclear area and cell-in-cell engulfment. Nuclear grading is a strong predictor of patient survival (29,30).
The nuclear atypia can be minute in MM, and in such cases difficult to distinguish from a reactive condition. When establishing a malignant condition: immunocytochemistry and ploidy analysis by fluorescence in situ hybridization (FISH) are commonly used. When accessible, also biomarker analyses and even electron microscopy (EM) can be helpful.
Immunocytochemistry (ICC)
Examination of routinely stained cell preparations is rarely—if ever—entirely diagnostic for MM. The diagnosis must always be based on a combination of cell morphology and outcome of adjuvant analyses, most often based on immunology. ICC will in most cases provide sufficient information for a specific and reliable diagnosis on the basis of which therapy can be initiated (20,31). A recent review presents the performance of the most commonly used epitopes (32). MM cells sometimes give a definite suspicion of this kind of cancer, but the atypia of these cells can also be mild and difficult to recognize as malignant. A frequent use of ICC is therefore advised as soon as the effusion is rich in mesothelial cells and cell groups.
There are two diagnostic challenges to diagnose a MM based on effusion cytology: first to diagnose malignancy and then to show that the malignant cells are mesothelial. Routine morphology is sometimes sufficient to establish malignancy, while adjuvant analyses are necessary in other cases. ICC is helpful in most of these cases. The antibodies used in effusion cytology are the same as those used in histological tumor tissue (3,27).
Several antibodies have been proposed to indicate benign/reactive conditions and distinguish them from malignant proliferations (33,34). Benign mesothelial cells express desmin (33) and BAP1, this expression often being lost early during carcinogenesis (35). A malignant effusion often contains scattered benign mesothelial cells that serve as internal positive controls. Using these epitopes, it must be considered that their reactivity can be affected by fixation (36). It may be that in formalin fixed and paraffin embedded cell-blocks BAP1 is preferred, while in alcohol fixed cytospin preparations desmin performs better with a sensitivity of 98% in our material. A combination of BAP1 and MTAP immunohistochemistry (37) and CDKN2A FISH (38) are very useful for separating benign from malignant mesothelial proliferations with sensitivity of 80% to 90% for epithelial MM in the pleura (39).
Presence of a distinct EMA reactivity distinguishes a malignant condition from reactive changes. Some expression of EMA can be seen also in reactive mesothelium and the combination of EMA and desmin in a double staining is helpful (Figure 1 and Figure 3: row 4; and Figure 2: row 3). The EMA reactive cells are in these cases less abundant than cells containing desmin and the reactivity is much weaker. In case routine cytomorphology and ICC is inconclusive regarding malignancy, other measures such as ploidy analysis by FISH can be useful (see below).
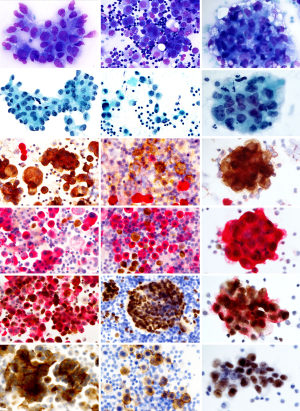
Once malignancy is established the second challenge is to demonstrate the mesothelial phenotype of the malignant cells. A large number of epitopes are available to solve this. A commonly recommended epitope for this purpose is Calretinin (40) showing strong, general reactivity in most MMs. Other useful markers of mesothelial lineage are Mesothelin, Podoplanin (D2-40) (41), Wilms tumor protein-1 (WT1), Cytokeratin 5, and HBME1 (42). They all show high sensitivity to MMs, providing an accurate phenotype diagnosis, when at least two of them show congruent results.
A common recommendation, when working with both histological and cytological material, is that the diagnosis should be supported by at least two markers in favor of the diagnosis and two markers contradicting MM. In case the outcome of ICC is incongruent this needs additional measures to warrant the diagnosis. In particular, antibodies labelling alien cell populations in the effusion are often used to exclude an MM. A metastatic carcinoma will most often express BerEp4/EpCAM and MOC31 antibodies and reactivity to monoclonal CEA will exclude MM (43). Thus, for example TTF1 and Napsin A will indicate lung adenocarcinoma, CDX-2 and CK20 are characteristic for colorectal carcinoma, ER/PGR are present in breast cancer and PAX8 together with Ca125 is seen in ovarian carcinomas (44). The latter epitope (Ca125) alone has no diagnostic importance in effusion cytology, since the protein binds to mesothelin present on the benign mesothelium. The interpretation of several of these immunoreactions is endorsed when performed as double stains. Helpful such combinations are BerEp4/Calretinin, Desmin/EMA, TTF1/NapsinA and PAX8/Ca125 (Figure 3).
Ploidy analysis by FISH
When routine cytomorphology and ICC is insufficient to establish malignancy, the analysis of ploidy, using FISH is recommended (45-49) for the definitive diagnosis.
The Urovysion® test (Abbot, Wiesbaden, Germany) will be diagnostic in this respect in the majority of cases (46,50). Two patterns indicate malignancy, either the homozygous deletion of 9p21, or gains of at least two centromeric signals. Care must, however, be taken with the latter criteria not to misinterpret tetraploidization during reactive proliferation as samples where all probes show 3–4 signals may well represent a benign proliferative condition. In this way aneuploidy can be proved with 80–90% sensitivity and 100% positive predictive value. A homozygous loss of the 9p21 signals has a 100% positive predictive value, as it is present only in malignant and premalignant lesions but never in reactive mesothelial cell proliferation. The finding of homozygous loss of the 9p21 signals is common in MM, although this is not specific for this tumor.
Other ancillary analyses
With the above-mentioned ancillary analyses, epithelioid and mixed type MM can be diagnosed by effusion cytology in the majority of cases. The sensitivity can be improved by using ultrastructure analysis and analyzing soluble biomarkers in effusion supernatant. These techniques are not available in every center, and will be only briefly described here.
Ultrastructure analysis by electron microscopy
Electron microscopy (EM) was previously considered gold standard for the diagnosis of MM, with well-known characteristics of MM cells (51). Cells from an effusion cell pellet are well suited for ultrastructural analysis, provided that a portion of the cell pellet is glutaraldehyde fixed early in the process (52).
Several findings such as long slender microvilli without signs of a glycocalyx indicate mesothelial lineage and other findings such as apical membranes baso-laterally or intracellular vesicles or neolumina are only seen in malignant conditions. The access to the technique and the time needed for preparing the ultrathin sections are the main limiting factors, EM being used when the other analyses remain inconclusive.
Soluble biomarkers
The ICC analyses demonstrate cell bound biomarkers. MMs also produce diagnostically useful soluble biomarkers that are secreted to the effusion supernatant or are released into the effusion fluid by cell decay. The analysis of such compounds can provide useful adjuncts to the diagnosis of MM. The two most established biomarkers are hyaluronan and mesothelin (53-58). Receiver operating characteristic (ROC) analysis of these two markers combined in a logistic model gives an area under curve (AUC) of 0.99 (58), indicating very high diagnostic accuracy. This analysis should, however, only be used as an adjuvant to the morphological diagnosis of MM.
Conclusions
Since the effusion often is the first diagnostic material available in patients with MM, cytology provides a possibility for earlier diagnosis. A diagnosis based already on the first effusion, would allow better effect of chemotherapy, since early treatment has shown to improve the clinical outcome (59). The cytological diagnosis of MM can be obtained with a high positive predictive value, applying the criteria defined in the guidelines in clinical routine. In our cohort, the median survival after a diagnosis of MM by effusion cytology was 20 months (53-58), while for those diagnosed by histology it was 12 months. It thus seems that cases that are possible to diagnose by cytology, including epithelioid and mixed subtypes, represent a sub-group of patients with a somewhat better prognosis. Therefore, in combination with the earlier diagnosis, a better effect of chemotherapy can be expected, providing therapy is initiated without delay in cases where effusion cytology is diagnostic. When using guideline criteria together with a liberal use of adjuvant analyses in 10 year’s clinical routine, the positive predictive value of a cytological diagnosis fulfilling these criteria was 100% (31). Thus, when a MM can be diagnosed in this way, this diagnosis is reliable and provides on its own sufficient ground for initiating therapy.
Acknowledgments
Funding: This paper was funded by The Swedish Cancer Society and The Cancer Society in Stockholm.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at available at
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See:
References
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379-87. [Crossref] [PubMed]
- Abdel-Rahman O. Global trends in mortality from malignant mesothelioma: Analysis of WHO mortality database (1994-2013). Clin Respir J 2018;12:2090-100. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordonez NG, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- O'Brien ME, Watkins D, Ryan C, et al. A randomised trial in malignant mesothelioma (M) of early (E) versus delayed (D) chemotherapy in symptomatically stable patients: the MED trial. Ann Oncol 2006;17:270-5. [Crossref] [PubMed]
- Abd Own S, Hoijer J, Hillerdahl G, et al. Effusion cytology of malignant mesothelioma enables earlier diagnosis and recognizes patients with better prognosis. Diagn Cytopathol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- Lechner JF, Tesfaigzi J, Gerwin BI. Oncogenes and tumor-suppressor genes in mesothelioma--a synopsis. Environ Health Perspect 1997;105 Suppl 5:1061-7. [PubMed]
- Lindholm PM, Salmenkivi K, Vauhkonen H, et al. Gene copy number analysis in malignant pleural mesothelioma using oligonucleotide array CGH. Cytogenet Genome Res 2007;119:46-52. [Crossref] [PubMed]
- Musti M, Kettunen E, Dragonieri S, et al. Cytogenetic and molecular genetic changes in malignant mesothelioma. Cancer Genet Cytogenet 2006;170:9-15. [Crossref] [PubMed]
- Churg A, Hwang H, Tan L, et al. Malignant mesothelioma in situ. Histopathology 2018;72:1033-8. [Crossref] [PubMed]
- Churg A, Galateau-Salle F, Roden AC, et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod Pathol 2020;33:297-302. [Crossref] [PubMed]
- Chapel DB, Schulte JJ, Berg K, et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod Pathol 2020;33:245-54. [Crossref] [PubMed]
- Matsumoto S, Hamasaki M, Kinoshita Y, et al. Morphological difference between pleural mesothelioma cells in effusion smears with either BAP1 loss or 9p21 homozygous deletion and reactive mesothelial cells without the gene alterations. Pathol Int 2019;69:637-45. [Crossref] [PubMed]
- Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264-9. [Crossref] [PubMed]
- Mäki-Nevala S, Sarhadi VK, Knuuttila A, et al. Driver Gene and Novel Mutations in Asbestos-Exposed Lung Adenocarcinoma and Malignant Mesothelioma Detected by Exome Sequencing. Lung 2016;194:125-35. [Crossref] [PubMed]
- Kang HC, Kim HK, Lee S, et al. Whole exome and targeted deep sequencing identify genome-wide allelic loss and frequent SETDB1 mutations in malignant pleural mesotheliomas. Oncotarget 2016;7:8321-31. [Crossref] [PubMed]
- Björkqvist AM, Tammilehto L, Nordling S, et al. Comparison of DNA copy number changes in malignant mesothelioma, adenocarcinoma and large-cell anaplastic carcinoma of the lung. Br J Cancer 1998;77:260-9. [Crossref] [PubMed]
- Zhou J, Sanchez-Vega F, Caso R, et al. Analysis of tumor genomic pathway alterations using broad-panel next-generation sequencing in surgically resected lung adenocarcinoma. Clin Cancer Res 2019;25:7475-84. [Crossref] [PubMed]
- Kato S, Tomson BN, Buys TP, et al. Genomic Landscape of Malignant Mesotheliomas. Mol Cancer Ther 2016;15:2498-507. [Crossref] [PubMed]
- Hmeljak J, Sanchez-Vega F, Hoadley KA, et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov 2018;8:1548-65. [Crossref] [PubMed]
- Segal A, Sterrett GF, Frost FA, et al. A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: results of a 20 year audit. Pathology 2013;45:44-8. [Crossref] [PubMed]
- Davidson B, Firat P, Michael CW. Serous effusions: etiology, diagnosis, prognosis, and therapy. London; New York: Springer, 2012.
- Bedrossian CWM. Malignant Effusions: A Multomodal Approach to Cytologic Diagnosis. New York: Igaku Shoin, 1994.
- Michael CW, Chieng D, Bedrossian CWM. The Cytohistology of the Serous Membranes. New York: Cambridge University Press, 2015.
- Tao L: Cytopathology of Malignant Effusions Chicago: ASCP Press, 1996.
- Kwee WS, Veldhuizen RW, Alons CA, et al. Quantitative and qualitative differences between benign and malignant mesothelial cells in pleural fluid. Acta Cytol 1982;26:401-6. [PubMed]
- Whitaker D. The cytology of malignant mesothelioma. Cytopathology 2000;11:139-51. [Crossref] [PubMed]
- Hjerpe A, Ascoli V, Bedrossian CW, et al. Guidelines for the cytopathologic diagnosis of epithelioid and mixed-type malignant mesothelioma. Complementary statement from the International Mesothelioma Interest Group, also endorsed by the International Academy of Cytology and the Papanicolaou Society of Cytopathology. Acta Cytol 2015;59:2-16. [Crossref] [PubMed]
- Matsumoto S, Nabeshima K, Kamei T, et al. Morphology of 9p21 homozygous deletion-positive pleural mesothelioma cells analyzed using fluorescence in situ hybridization and virtual microscope system in effusion cytology. Cancer Cytopathol 2013;121:415-22. [Crossref] [PubMed]
- Rosen LE, Karrison T, Ananthanarayanan V, et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod Pathol 2018;31:598-606. [Crossref] [PubMed]
- Kimura N, Dota K, Araya Y, et al. Scoring system for differential diagnosis of malignant mesothelioma and reactive mesothelial cells on cytology specimens. Diagn Cytopathol 2009;37:885-90. [Crossref] [PubMed]
- Hjerpe A, Abd-Own S, Dobra K. Cytopathologic Diagnosis of Epithelioid and Mixed-Type Malignant Mesothelioma: Ten Years of Clinical Experience in Relation to International Guidelines. Arch Pathol Lab Med 2018;142:893-901. [Crossref] [PubMed]
- Louw A, Badiei A, Creaney J, et al. Advances in pathological diagnosis of mesothelioma: what pulmonologists should know. Curr Opin Pulm Med 2019;25:354-61. [Crossref] [PubMed]
- Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology 2003;43:231-8. [Crossref] [PubMed]
- Churg A, Sheffield BS, Galateau-Salle F. New markers for separating benign from malignant mesothelial proliferations: are we there yet? Arch Pathol Lab Med 2016;140:318-21. [Crossref] [PubMed]
- Önder S, Ozogul E, Koksal D, et al. Diagnostic value of BRCA1-associated protein-1, glucose transporter-1 and desmin expression in the discrimination between reactive mesothelial proliferation and malignant mesothelioma in tissues and effusions. Cytopathology 2019;30:592-600. [Crossref] [PubMed]
- Parham DM, Webber B, Holt H, et al. Immunohistochemical study of childhood rhabdomyosarcomas and related neoplasms. Results of an Intergroup Rhabdomyosarcoma study project. Cancer 1991;67:3072-80. [Crossref] [PubMed]
- Kinoshita Y, Hamasaki M, Yoshimura M, et al. A combination of MTAP and BAP1 immunohistochemistry is effective for distinguishing sarcomatoid mesothelioma from fibrous pleuritis. Lung Cancer 2018;125:198-204. [Crossref] [PubMed]
- Hwang HC, Sheffield BS, Rodriguez S, et al. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the Diagnosis of Malignant Mesothelioma in Effusion Cytology Specimens. Am J Surg Pathol 2016;40:120-6. [Crossref] [PubMed]
- Siddiqui MT, Schmitt F, Churg A. Proceedings of the American Society of Cytopathology companion session at the 2019 United States and Canadian Academy of Pathology Annual meeting, part 2: effusion cytology with focus on theranostics and diagnosis of malignant mesothelioma. J Am Soc Cytopathol 2019;8:352-61. [Crossref] [PubMed]
- Ordóñez NG. Value of calretinin immunostaining in diagnostic pathology: a review and update. Appl Immunohistochem Mol Morphol 2014;22:401-15. [Crossref] [PubMed]
- Ordóñez NG. Value of podoplanin as an immunohistochemical marker in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol 2014;22:331-47. [Crossref] [PubMed]
- Ordóñez NG. Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: a review and update. Hum Pathol 2013;44:1-19. [Crossref] [PubMed]
- Dejmek A, Hjerpe A. Carcinoembryonic antigen-like reactivity in malignant mesothelioma. A comparison between different commercially available antibodies. Cancer 1994;73:464-9. [Crossref] [PubMed]
- Ordóñez NG. Value of PAX8, PAX2, claudin-4, and h-caldesmon immunostaining in distinguishing peritoneal epithelioid mesotheliomas from serous carcinomas. Mod Pathol 2013;26:553-62. [Crossref] [PubMed]
- Factor RE, Dal Cin P, Fletcher JA, et al. Cytogenetics and fluorescence in situ hybridization as adjuncts to cytology in the diagnosis of malignant mesothelioma. Cancer 2009;117:247-53. [PubMed]
- Flores-Staino C, Darai-Ramqvist E, Dobra K, et al. Adaptation of a commercial fluorescent in situ hybridization test to the diagnosis of malignant cells in effusions. Lung Cancer 2010;68:39-43. [Crossref] [PubMed]
- Illei PB, Ladanyi M, Rusch VW, et al. The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer 2003;99:51-6. [Crossref] [PubMed]
- Savic S, Franco N, Grilli B, et al. Fluorescence in situ hybridization in the definitive diagnosis of malignant mesothelioma in effusion cytology. Chest 2010;138:137-44. [Crossref] [PubMed]
- Hiroshima K, Wu D, Hasegawa M, et al. Cytologic Differential Diagnosis of Malignant Mesothelioma and Reactive Mesothelial Cells With FISH Analysis of p16. Diagn Cytopathol 2016;44:591-8. [Crossref] [PubMed]
- Wan C, Shen YC, Liu MQ, et al. Diagnostic value of fluorescence in situ hybridization assay in malignant mesothelioma: a meta-analysis. Asian Pac J Cancer Prev 2012;13:4745-9. [Crossref] [PubMed]
- Wang NS. Electron microscopy in the diagnosis of pleural mesotheliomas. Cancer 1973;31:1046-54. [Crossref] [PubMed]
- Domínguez-Malagón H, Cano-Valdez AM, Gonzalez-Carrillo C, et al. Diagnostic efficacy of electron microscopy and pleural effusion cytology for the distinction of pleural mesothelioma and lung adenocarcinoma. Ultrastruct Pathol 2016;40:254-60. [Crossref] [PubMed]
- Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612-6. [Crossref] [PubMed]
- Creaney J, Yeoman D, Naumoff LK, et al. Soluble mesothelin in effusions: a useful tool for the diagnosis of malignant mesothelioma. Thorax 2007;62:569-76. [Crossref] [PubMed]
- Pass HI, Wali A, Tang N, Ivanova A, et al. Soluble mesothelin-related peptide level elevation in mesothelioma serum and pleural effusions. Ann Thorac Surg 2008;85:265-72; discussion 272. [Crossref] [PubMed]
- Creaney J, Dick IM, Segal A, et al. Pleural effusion hyaluronic acid as a prognostic marker in pleural malignant mesothelioma. Lung Cancer 2013;82:491-8. [Crossref] [PubMed]
- Fujimoto N, Gemba K, Asano M, et al. Hyaluronic acid in the pleural fluid of patients with malignant pleural mesothelioma. Respir Investig 2013;51:92-7. [Crossref] [PubMed]
- Mundt F, Nilsonne G, Arslan S, et al. Hyaluronan and N-ERC/mesothelin as key biomarkers in a specific two-step model to predict pleural malignant mesothelioma. PLoS One 2013;8:e72030. [Crossref] [PubMed]
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic Factors in the Treatment of Malignant Pleural Mesothelioma at a Large Tertiary Referral Center. J Thorac Oncol 2007;2:957-65. [Crossref] [PubMed]


