HER2-D16 oncogenic driver mutation confers osimertinib resistance in EGFR mutation-positive non-small cell lung cancer
Epidermal growth factor receptor (EGFR) mutations are the most frequent drivers of tumor development among patients with non-small cell lung cancer (NSCLC) in Asian-Pacific countries. Several phase 3 trials have demonstrated that first-generation (gefitinib and erlotinib) and second-generation (afatinib and dacomitinib) EGFR-tyrosine kinase inhibitors (TKIs) exhibit superior efficacy to standard platinum-based chemotherapy for treating patients with EGFR mutation-positive advanced NSCLC (1-3). Despite the remarkable success of EGFR-TKIs in clinical settings, with an objective response rate of 60–70%, the emergence of resistance remains a major limitation to therapeutic efficacy, with the average progression-free survival (PFS) ranging from 9 to 15 months (4). Development of the EGFR-T790M mutation in EGFR exon 20, which prevents the binding of first- and second-generation EGFR-TKIs to the ATP binding site, has been reported as the most common resistance mechanism (5). However, the third-generation EGFR-TKI osimertinib was developed for clinical use to overcome resistance (6). Currently, osimertinib, a mutant selective, irreversible EGFR-TKI, is approved for treating patients with EGFR-T790M-positive advanced NSCLC following treatment with first- and second-generation EGFR-TKIs and has been also approved as a first-line therapy for patients with EGFR mutation-positive advanced NSCLC regardless of their T790M mutation status (7). As an initial therapy, osimertinib resulted in a median PFS of 18.9 months (7). After the failure of previous EGFR-TKI therapy with EGFR-T790M mutation, osimertinib exhibited significantly greater efficacy than platinum-pemetrexed therapy. The median PFS was significantly longer with osimertinib than with platinum-pemetrexed (10.1 vs. 4.4 months). The objective response rate was significantly better with osimertinib than with platinum-pemetrexed (71% vs. 31%) (8). Therefore, osimertinib was approved for treating patients with EGFR-T790M-positive NSCLC following progression during prior EGFR-TKI treatment. However, all patients ultimately developed resistance to osimertinib in both in the first-line treatment setting and as a salvage therapy for EGFR-T790M-positive NSCLC. The mechanisms underlying resistance to osimertinib include a wide spectrum of aberrations, reflecting the molecular heterogeneity of NSCLC tumors.
Hsu et al. (9) recently described a human epidermal growth factor 2 (HER2) exon 16-skipping mutation in a patient with NSCLC also harboring EGFR-L858R/T790M mutations who acquired resistance to osimertinib. To determine the underlying mechanism linking HER2 mutation with drug resistance, the researchers used an in vitro model of H1975 cells stably expressing high levels of HER2 accompanied by an exon 16-skipping deletion (designated HER2-D16). The study showed that afatinib, a pan-HER inhibitor, overcame HER2-D16 induced osimertinib-resistance by using a combination of osimertinib in a preclinical model of stably expressing HER2-D16-H1975 cells. Hsu et al. showed that combination treatment with osimertinib and afatinib, which inhibits HER2 activation, suppressed the proliferation of HER2-D16-mutant cells. This study supports HER2-D16 emergence causing bypass signals in clinical samples, providing insight into targeted treatment for HER2-mutated solid tumors, including NSCLC.
HER2 (also known as erbB-2/neu) is a member of the erbB receptor tyrosine kinase family and is encoded by ERBB2, a major proliferative driver that activates downstream signaling through the PI3K-AKT and MEK-ERK pathways. No ligand has been described for this receptor, which is activated by homodimerization or heterodimerization with other members of the erbB family. HER2 alterations have been identified as oncogenic drivers and potential therapeutic targets in lung cancers (10,11); HER2 mutations and amplification have been reported in approximately 2–3% and 2–5% of lung adenocarcinomas, respectively (12,13). Interestingly, HER2 mutation is more commonly associated with a selected subgroup of women and never-smokers harboring tumors with an adenocarcinoma histology (14). Similar findings were reported for subpopulations of patients with lung cancers harboring EGFR mutations (15), suggesting that similar genetic factors, carcinogens, or environmental factors affect the occurrence of mutations in both EGFR and HER2. However, no specific therapeutics targeting HER2 amplification or mutations have been developed.
The resistance mechanisms to EGFR-TKIs can be broadly divided into five groups: secondary mutations in EGFR (e.g., T790M, C797S), bypass signaling activation (e.g., HER2, AXL, cMET), activation of downstream molecules (e.g., PIK-3CA mutation, PTEN loss, BRAF mutation), phenotypic transformation (e.g., small cell lung cancer, epithelial-mesenchymal transition), and resistance to apoptotic cell death (e.g., BIM polymorphism) (16). An increased HER2 copy number has also been associated with a poor therapeutic response to EGFR-TKIs in patients with lung cancer (17). Using clinical samples and preclinical models with acquired resistance to EGFR-TKIs, HER2 amplification was detected along with the loss of EGFR-T790M mutation, indicating that HER2 amplification and EGFR-T790M are mutually exclusive (18,19). HER2 amplification was detected in approximately 10% of cases exhibiting EGFR-TKI resistance but in only 3% of cases without prior EGFR-TKI treatment (20). HER2 mutation causing an exon 20 insertion was detected in the circulating tumor DNA of the plasma from a patient who acquired resistance to osimertinib (21). The most common HER2 mutation consists of a 12-base pair (bp) insertion in exon 20, resulting in addition of the amino acid residues YVMA. However, HER2 amplification does not typically coexist with HER2 mutations (20).
Hsu et al. first identified the novel HER2-D16 mutation as a deletion in exon 16 in a patient with EGFR-L858R/T790M-positive NSCLC (9). Alternatively, in breast cancer, the HER2-D16 mutation was recognized as a crucial driver of aggressive behaviors of HER2-positive tumors and was significantly associated with locally disseminated lymph nodes-positive breast cancer (22). Moreover, amplified HER2-D16-driven oncogenic signals were associated with the downstream oncogenic SRC signal transduction pathway. This HER2-D16–SRC axis has also been shown to be activated in mammary adenocarcinomas from genetically engineered cell lines and in HER2-positive breast cancer tissues from patients (22,23). Therefore, activated SRC is the key surrogate marker of HER2-positive breast cancer, which acts downstream of the oncogenic HER2-D16 signal. However, in this new study in a HER2-D16-expressing NSCLC cell model, Hsu et al. (9) demonstrated that signal transduction of HER2-D16-driven resistance to osimertinib occurred independently of SRC. This model was established from H1975 cells expressing EGFR-L858R/T790M, which were then transfected with plasmids encoding HER2-D16, followed by the selection of stable clones, resulting in cells expressing the dual driver mutations EGFR-L858R/T790M and HER2-D16. These dual driver mutations cooperatively influenced the downstream signal transduction pathway in this model. All HER2 mutations were mutually exclusive with other driver mutations in EGFR, KRAS, BRAF, NRAS, PI3KCA, MEK, and AKT, as well as ALK rearrangements. Accordingly, whether EGFR-L858R and EGFR-T790M mutations coexist with HER2-D16 in the same tumor remained unclear. The EGFR-L858R/T790M and HER2-D16 mutations may have existed in different tumor cells in this patient.
Hsu and colleagues further observed loss of EGFR-T790M following osimertinib treatment from cell-free DNA of the patient, whereas the expression level of HER2-D16 was increased. Osimertinib treatment failed in this patient. Interestingly, the expression of EGFR-T790M and HER2-D16 were exclusive and showed an opposite relationship with the response to osimertinib, whereas the expression level of EGFR-L858R did not change during osimertinib treatment based on plasma cell-free DNA analysis. A similar loss of the target EGFR mutation by EGFR-TKI treatment has been reported in both preclinical and clinical studies (24). We also reported loss of an EGFR-activating mutation (15-bp deletion in EGFR exon 19: EGFR-ex19del) in an afatinib-resistant cell line (25). Similar results were reported for the loss of mutant alleles from gefitinib- and erlotinib-resistant PC-9, HCC827, and 11-18 cells, which also involved bypass of the signal transduction pathway to HER2, ERBB3, or IGF1R, or induction of epithelial-mesenchymal transition with stem cell-like properties (26,27). Moreover, loss of EGFR-activating mutations was observed in clinical samples from patients who were refractory to EGFR-TKI treatment (26). Recently, wild-type EGFR amplification was observed in PC-9 cells resistant to the third-generation EGFR-TKI rociletinib harboring an exon 19 deletion in EGFR and in clinical tissue samples from patients after the failure of rociletinib treatment (28,29).
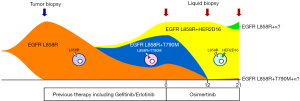
In the study by Hsu et al. (9), the patient’s right lung tumor harbored the EGFR-L858R mutation, and thus gefitinib treatment was initiated; however, his disease progressed within only 4 months of treatment. The regimen was then switched to cisplatin-pemetrexed chemotherapy, erlotinib, paclitaxel, gemcitabine, and vinorelbine continuously. Despite treatment, a malignant pleural effusion developed, which showed an EGFR-L858R mutation in exon 21 and EGFR-T790M mutation in exon 20. These two EGFR mutations along with the HER2-D16 mutation were detected in plasma cell-free DNA before osimertinib treatment. This suggests the existence of at least three types of clonal cells in tumors expressing EGFR-L858R, EGFR-L8578R/T790M, and EGFR-L858R + HER2-D16 (Figure 1). Thus, osimertinib treatment selected tumor cells expressing EGFR-L858R + HER2-D16, as these cells were not effectively suppressed by the treatment, whereas cells expressing EGFR-L858R and EGFR-L858R/T790M were decreased. This may further explain why the cells expressing EGFR-L858R were not decreased based on analysis of plasma cell-free DNA. Specifically, prior to gefitinib treatment, most tumor cells may have harbored the EGFR-L858R mutation; however, during treatment with the first-generation EGFR-TKIs gefitinib and erlotinib, the tumor cells acquired the EGFR-T790M along with the EGFR-L858R mutation because of the adaptation of selective pressure or spontaneous evolutionary emergence. Therefore, based on cell-free DNA analysis, at the time of initial treatment of osimertinib, it is possible that at least three types of EGFR/HER2-mutated tumor cells may have co-existed in the same tumor, including EGFR-L858R, EGFR-L858R/T790M, and EGFR-L858R + HER2-D16. Thus, osimertinib completely suppressed tumor cells expressing EGFR-L858R and EGFR-L858R/T790M, leaving only those expressing EGFR-L858R + HER2-D16. Although these EGFR-L858R and HER2-D16 driver mutations may have exclusive effects and mechanisms, they may exist in the same tumor cells. Indeed, the expression levels of EGFR-L858R and HER2-D16 were very similar, with 79% and 80% positivity at 12 weeks and 82% and 70% positivity at 21 weeks of osimertinib treatment, respectively, further supporting that these mutations coexisted in the same tumor cells.
Nakatani et al. (30) reported that treatment with rociletinib, a third generation EGFR-TKI, suppressed the proliferation of afatinib-resistant PC-9 cells expressing EGFR ex19del/T790M. Rociletinib-resistant cell lines were then established by 10–12-month exposure to progressively increasing concentrations of rociletinib. Allelic quantitative distribution analysis using EGFR mutation-specific droplet digital PCR showed that the EGFR ex19del and EGFR-T790M alleles were substantially decreased in these rociletinib-resistant cell lines (named RocR1 and RocR2 cells). Because the third-generation EGFR-TKIs rociletinib and osimertinib are EGFR mutation-selective inhibitors, suppression of the expression of the target EGFR ex19del/T790M along with amplification of wild-type EGFR in RocR1 and RocR2 cells led to the use of bypass signal pathway(s) for cell survival. Therefore, the lack of a change in the expression level of EGFR-L858R during osimertinib treatment observed by Hsu et al. (9) is intriguing, as the expression of EGFR-T790M was attenuated whereas HER2-D16 expression was increased. These results further suggest that EGFR-L858R coexisted with both EGFR-T790M and HER2-D16 in the same tumor cells in the patient. These findings have clinical applications, highlighting the importance of repeated analyses of EGFR mutation status during EGFR-TKI treatment.
This study further demonstrated that suppression of HER2D16 activation by afatinib, a pan-HER inhibitor, in combination with suppression of EGFR-L858R/T790M by osimertinib had a synergistic effect on the resistance conferred by these two mutations. Interestingly, the inhibitory effect of trastuzumab for HER2D16 in breast cancer is controversial because conflicting results have been observed between in vitro and in vivo studies (22,23). In breast cancer, HER2-targeted therapies have significantly improved the survival of patients with HER2-positive tumors as an adjuvant/neoadjuvant treatment and in metastatic cases. In gastric cancer, HER2-targeted therapy of a trastuzumab-based regimen was also approved for HER2-positive, previously untreated metastatic gastric cancer (31). In these cases, “HER2-positive” is determined based on an immunohistochemical staining score of 3+ in biopsy or surgically resected tumor samples. Combination treatment of trastuzumab and pertuzumab, as dual anti-HER2 humanized monoclonal antibodies, with chemotherapy is recommended as a first-line therapy for metastatic breast cancer. Other HER2-target therapies such as the HER2 kinase inhibitor lapatinib and antibody-drug conjugate (ADC) trastuzumab emtansine (T-DM1) can be used as subsequent treatment options. However, there is currently no approved targeted therapy for solid tumors with HER2 mutations, including breast cancer, NSCLC, gastric cancer, or colon cancer. Moreover, patients with HER2 mutations show a worse prognosis patients with lung adenocarcinomas containing other oncogenic drivers (32). Therefore, effective therapeutics for patients with HER2 mutations are required.
Recently, the clinical effects of the novel ADC trastuzumab deruxtecan (T-DXd; DS-8201a) were reported in a phase I trial of patients with HER2-expressing non-breast and non-gastric cancer or HER2-mutant solid tumors (33). T-DXd is a humanized anti-HER2 antibody with a cleavable, peptide-based linker, and potently inhibits topoisomerase I. In this trial, patients with HER2-mutant NSCLC had more pronounced tumor shrinkage than those with wild-type HER2-expressing NSCLC, with an objective response rate of 72.7% (8/11) and median PFS of 11.3 months (95% confidence interval 8.1–14.3 months). The safety profile was generally acceptable. Among the 11 patients with NSCLC with HER2 mutations enrolled in the study, eight had kinase domain mutations, two had transmembrane domain mutations, and one had extracellular domain mutations; however, no patient with NSCLC harbored the HER2-D16 mutation. Li et al. (34) demonstrated that HER2-activating mutations facilitated receptor ubiquitination and internalization, resulting in enhanced sensitivity to anti-HER2 ADCs such as T-DM1 and T-DXd in lung cancer based on preclinical models and clinical samples. Furthermore, a pan-HER irreversible inhibitor such as neratinib or afatinib enhanced internalization of the HER2-ADC complex by increasing HER2 ubiquitination through inhibition of HSP90 activity. In vivo efficacy evaluation showed that both T-DM1 alone and the combination of T-DM1 and neratinib induced marked tumor regression in an ERBB2 S310F mutant PDX model, although the effect was more durable with combined treatment. However, there are no current clinical trials evaluating the clinical benefit of the combination of T-DM1 and a pan-HER irreversible inhibitor in patients with ERBB2-mutant lung cancer.
Overall, Hsu et al. (9) demonstrated the potential of combining osimertinib and afatinib for treating NSCLC with EGFR-L858R/T790M and HER2D16 mutations in a preclinical model. As mentioned above, this in vitro H1975 cell model, expressing both EGFR-L858R/T790M + HER2D16, is an exceptional case and may differ from the patient’s mutation profile, indicating that the tumor expressed EGFR-L858R + HER2D16 in plasma cell-free DNA analysis. This is consistent with the exclusive relationship between EGFR-T790M and HER2 mutation/amplification. It may be useful to treat the model of EGFR-L858R + HER2D16 with afatinib as a single agent, providing additional potential therapeutics for patients with EGFR mutation-positive NSCLC. This new study along with the findings summarized should be further evaluated by HER2 mutation analysis and to evaluate HER2 inhibitor strategies using optimal drugs and drug combinations to enable precision medicine for HER2-mutated lung cancer using a molecular-targeted and rational approach. Furthermore, during treatment with EGFR-TKIs, repeated analysis of oncogenic driver mutations using plasma cell-free DNA would be essential for ensuring the effectiveness of therapeutics even after acquiring resistance in patients with EGFR mutation-positive NSCLC.
Acknowledgments
We thank the members of the Advanced Cancer Translational Research Institute, and Division of Allergology and Respiratory Medicine, Department of Medicine, Showa University School for their thoughtful discussions and helpful advice, as well as Editage for their assistance with English-language editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-578). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018;15:694-708. [Crossref] [PubMed]
- Lim SM, Syn NL, Cho BC, et al. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev 2018;65:1-10. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Hsu CC, Liao BC, Liao WY, et al. Exon 16-Skipping HER2 as a Novel Mechanism of Osimertinib Resistance in EGFR L858R/T790M-Positive Non-Small Cell Lung Cancer. J Thorac Oncol 2020;15:50-61. [Crossref] [PubMed]
- Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910-8. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Hirsch FR, Franklin WA, Veve R, et al. HER2/neu expression in malignant lung tumors. Semin Oncol 2002;29:51-8. [Crossref] [PubMed]
- Heinmöller P, Gross C, Beyser K, et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res 2003;9:5238-43. [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [Crossref] [PubMed]
- Yamaoka T, Ohba M, Ohmori T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int J Mol Sci 2017;18:2420. [Crossref] [PubMed]
- Cappuzzo F, Varella-Garcia M, Shigematsu H, et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol 2005;23:5007-18. [Crossref] [PubMed]
- Takezawa K, Pirazzoli V, Arcila ME, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922-33. [Crossref] [PubMed]
- Planchard D, Loriot Y, Andre F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 2015;26:2073-8. [Crossref] [PubMed]
- Li BT, Ross DS, Aisner DL, et al. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J Thorac Oncol 2016;11:414-9. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Mitra D, Brumlik MJ, Okamgba SU, et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther 2009;8:2152-62. [Crossref] [PubMed]
- Castagnoli L, Iezzi M, Ghedini GC, et al. Activated d16HER2 homodimers and SRC kinase mediate optimal efficacy for trastuzumab. Cancer Res 2014;74:6248-59. [Crossref] [PubMed]
- Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019;121:725-37. [Crossref] [PubMed]
- Yamaoka T, Ohmori T, Ohba M, et al. Distinct Afatinib Resistance Mechanisms Identified in Lung Adenocarcinoma Harboring an EGFR Mutation. Mol Cancer Res 2017;15:915-28. [Crossref] [PubMed]
- Tabara K, Kanda R, Sonoda K, et al. Loss of activating EGFR mutant gene contributes to acquired resistance to EGFR tyrosine kinase inhibitors in lung cancer cells. PLoS One 2012;7:e41017. [Crossref] [PubMed]
- Shien K, Toyooka S, Yamamoto H, et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res 2013;73:3051-61. [Crossref] [PubMed]
- Nukaga S, Yasuda H, Tsuchihara K, et al. Amplification of EGFR Wild-Type Alleles in Non-Small Cell Lung Cancer Cells Confers Acquired Resistance to Mutation-Selective EGFR Tyrosine Kinase Inhibitors. Cancer Res 2017;77:2078-89. [Crossref] [PubMed]
- Piotrowska Z, Niederst MJ, Karlovich CA, et al. Heterogeneity Underlies the Emergence of EGFRT790 Wild-Type Clones Following Treatment of T790M-Positive Cancers with a Third-Generation EGFR Inhibitor. Cancer Discov 2015;5:713-22. [Crossref] [PubMed]
- Nakatani K, Yamaoka T, Ohba M, et al. KRAS and EGFR Amplifications Mediate Resistance to Rociletinib and Osimertinib in Acquired Afatinib-Resistant NSCLC Harboring Exon 19 Deletion/T790M in EGFR. Mol Cancer Ther 2019;18:112-26. [Crossref] [PubMed]
- Meric-Bernstam F, Johnson AM, Dumbrava EEI, et al. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin Cancer Res 2019;25:2033-41. [Crossref] [PubMed]
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017;123:4099-105. [Crossref] [PubMed]
- Tsurutani J, Iwata H, Krop I, et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov 2020;10:688-701. [Crossref] [PubMed]
- Li BT, Michelini F, Misale S, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov 2020;10:674-87. [Crossref] [PubMed]