Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab
Introduction
The current standard first-line treatment in advanced non-small cell lung cancer (aNSCLC) with programmed cell death-ligand 1 (PD-L1) tumour proportion score (TPS) of at least 50% includes pembrolizumab monotherapy (1), with a 3-year overall survival (OS) rate of 43.7% as compared to 24.9% with chemotherapy, supported by durable disease responses (2). However, pembrolizumab monotherapy is being challenged by the addition of chemotherapy (3) or a different immune-checkpoint inhibitor (ICI) (4). Hence, there is a need for reliable biomarkers to predict patients’ outcome and disease response following pembrolizumab monotherapy (5,6).
The neutrophil-to-lymphocyte ratio (NLR) is a surrogate for tumour-associated inflammation and likely represents the frequency and activity of myeloid-derived suppressor cells (MDSCs), that hinder T-cell proliferation and expansion (7). Lactate dehydrogenase (LDH) has been investigated as a potential inflammatory biomarker in patients with cancer and is associated with poor outcomes in several cancer types (8). In aNSCLC, high NLR and its combination with high LDH, namely the immune prognostic index (LIPI), were correlated with worse outcomes for ICIs, but not for chemotherapy (8,9).
We explored the NLR in combination with PD-L1 or LDH as possible biomarkers for high PD-L1 aNSCLC with first-line pembrolizumab.
We present the following analysis in accordance with the REMARK Guideline (10) (available at http://dx.doi.org/10.21037/tlcr-19-583).
Patients and methods
The study analysis aimed to explore the prognostic value of low NLR, high PD-L1 TPS level and normal LDH, and of the combination of low NLR with high PD-L1 or normal LDH, in high PD-L1 aNSCLC treated with first-line pembrolizumab treated in five European Centers (three in Italy, one in the UK, one in Switzerland), as possible tools to identify patients with favourable outcome.
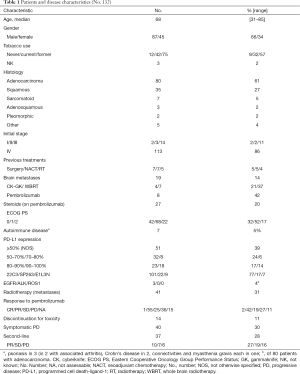
Patients aged more than 18 years, with histologically confirmed aNSCLC, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) ≤2, PD-L1 TPS >50%, treated with first-line pembrolizumab were retrospectively assessed. The analysis followed the principles outlined in the Declaration of Helsinki (as revised in 2013) for all human or animal experimental investigations. The analysis involved a real-world series of patients treated according to the clinical practice; ethical approval was waived because all patients in each Center signed a specific consent form for their data collection and sharing with other Institutions.
Three baseline parameters, from fresh routine blood or histopathological samples, were retrospectively analyzed: (I) the NLR, or the ratio between absolute neutrophils and lymphocytes; (II) the PD-L1 TPS level on the histological sample; (III) the blood level of LDH (in units/litre). The blood for NLR and LDH values was yielded within seven days from the ICI treatment start and routinely analyzed by local laboratories. PD-L1 was assessed by the platform and antibody used in each Center (see Table 1).
Full table
The cut-offs for NLR, PD-L1 and LDH were identified by receiver operating characteristic (ROC) curves.
For the combined biomarkers analysis patients with low NLR were grouped with those with available and high PD-L1 or available and normal LDH, with a possible overlapping between these two cohorts, or high PD-L1 and normal LDH.
The prognostic role in OS and progression-free survival (PFS) was assessed by the two-sided log-rank test; the association with response rate (RR) and progressive disease (PD) by the 2-tailed Fisher exact test.
RR, including complete response (CR) and partial response (PR), and PD, as the best response to the treatment, were assessed by the Response Evaluation Criteria in Solid Tumours (RECIST) criteria version 1.1 (11).
Statistical significance was investigated by Chi-square test and 2-tailed Fisher exact tests with an acceptable significance value of P<0.05. The OS and PFS were calculated from the date of ICI treatment start until death or last date of follow-up, and of progression or death from any cause, respectively, were estimated using the Kaplan-Meier, reported as medians with confidence limits (95% CI) and compared using two-sided log-rank test, with an acceptable significance value of P<0.05. Patients who did not have events at the time of the analysis have been censored. Considering an expected 2-year OS of 52% in this patient population (2) and aimed at finding a difference in the 2-year OS of 25% with the combined biomarker analysis, the required sample size was 113 patients.
Results
Patients’ characteristics are summarized in Table 1. One hundred thirty-two patients treated with pembrolizumab from December 2016 to June 2019 were analyzed. Twenty-two patients (17%) discontinued the treatment, 11 due to toxicity; 6 of them because of pneumonia and one due to worsening of pre-existent myasthenia gravis.
NLR was available for all patients, while PD-L1 level and LDH were available only for 81 (61%) and 85 (64%) patients, respectively.
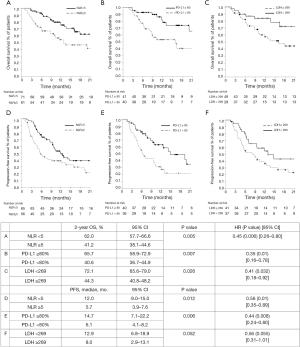
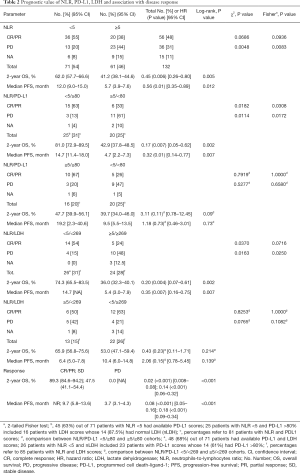
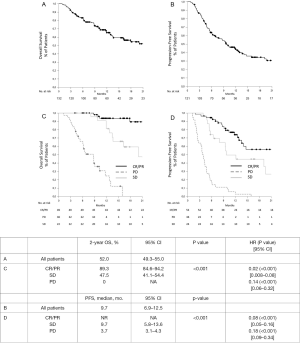
With a median follow-up of 16.3 months (95% CI: 15.0–17.7), the 2-year OS of all patients was 52.0% (95% CI: 49.3–55.0) and the median PFS 9.7 months (95% CI: 6.9–12.5) (see Figure S1A,B). Both OS and PFS were significantly associated with the disease response, CR/PR (2-year OS 89.3%, median PFS not reached) vs. SD (47.5%, 9.7 months) vs. PD (0%, 3.7 months) (P<0.001 for both, respectively) (see Table 2 and Figure S1C,D).
Full table
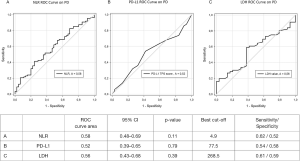
NLR, PD-L1 and LDH cut-offs by ROC curves were 4.9, 77.5% and 268.5, respectively (see Figure S2).
Seventy-one patients (54%) had NLR <5, 45 (63%) and 48 (68%) of them had available PD-L1 and LDH scores, respectively. Twenty-five patients out of the 81 with NLR and PD-L1 scores (31%) had NLR <5 and PD-L1 >80% and included 16 patients with LDH scores whose 14 (87.5%) had normal LDH (nLDH); 26 patients out of 85 patients with NLR and LDH scores (31%) had NLR <5 and nLDH and included 23 patients with PD-L1 scores whose 14 (61%) had PD-L1 >80%.
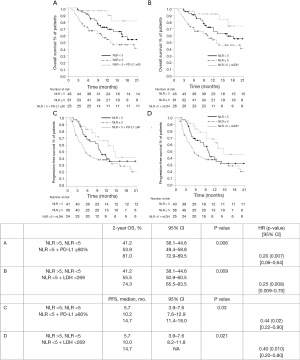
NLR <5, PD-L1 ≥80% and LDH <269 significantly associated with both OS and PFS (see Figure 1), with 2-year OS of 62.0% versus 41.2% and median PFS of 12.0 versus 5.7 months, for patients with NLR <5 as compared to those with NLR >5 [P=0.005, hazard ratio (HR) 0.45 and P=0.012, HR 0.56, respectively); 65.7% versus 40.6% and 14.7 versus 6.1 months, for PD-L1 ≥80% versus PD-L1 <80% (P=0.007, HR 0.35, and P=0.006, HR 0.44, respectively); and 72.1% versus 44.3% and 12.9 versus 8.0 months, for LDH <269 versus LDH ≥269 (P=0.026, HR 0.41, and P=0.052, HR 0.56, respectively).
Data on outcomes by NLR, PD-L1 and LDH cut-offs are reported in Table 2 and Figure 2. Better 2-year OS and PFS were seen in patients with low NLR <5 and high PD-L1≥80% as compared to those with high NLR and low PD-L1 (81.0% vs. 42.9%, P=0.002, HR 0.17, and median of 14.7 vs. 4.7 months, P=0.007, HR 0.32, respectively); a trend toward better OS but not PFS was observed in patients with low NLR and high PD-L1 as compared to those with high NLR and high PD-L1 (81.0% vs. 47.7%, P=0.09, HR 3.11, and median of 14.7 vs. 19.2 months, P=0.73, HR 1.18, respectively). Better 2-year OS and PFS were seen in patients with low NLR <5 and nLDH <269 as compared to those with high NLR and high LDH (74.3% vs. 36.0%, P=0.002, HR 0.20, and median of 14.7 vs. 5.4 months, P=0.007, HR 0.35, respectively); a trend toward better OS and PFS was observed in patients with low NLR and nLDH as compared to those with high NLR and low PD-L1 (74.3% vs. 65.9%, P=0.214, HR 0.43, and median of 14.7 vs. 6.4 months, P=0.139, HR 2.06, respectively).
As far as the disease response is concerned, low NLR <5 significantly associated with PD (P=0.008) but not RR (P=0.09); low NLR <5 with high PD-L1 >80% both RR (P=0.03) and PD (P=0.02), low NLR <5 with nLDH with PD but not RR (P=0.025 and P=0.07, respectively).
Discussion
We found that low NLR <5, high PD-L1 >80% and LDH <269, especially when low NLR <5 was combined with one of the other two factors, significantly associated with favourable outcomes following first-line pembrolizumab monotherapy in aNSCLC. This underlines the essential role of tumour inflammation and microenvironment (12).
Differently from other reported series, the cut-offs we used for NLR, PD-L1 and LDH were ROC-based and quantitative. This could raise the issue of their external validity, of the need for their validation or just of comparison with those currently reported. As far as the NLR is concerned, our ROC-based cut-off of 5 mirrored what has already been reported in patients with NSCLC (9) and previously validated in those with metastatic melanoma treated with ipilimumab (13). In the LIPI score for NSCLC patients (8), the NLR cut-off used was instead 3, based on a larger and updated series of patients with metastatic melanoma treated with ipilimumab (14). Regarding LDH, there is no current standard cut-off. The upper limit of normal (ULN) value according to the limit of each center was for example adopted for the LIPI score, where the median LDH value of all patients was 248.5 (8). In the largest series of patients with metastatic melanoma treated with pembrolizumab an LDH value of at least 2.5 times ULN resulted as an independent prognostic value (15), while in another series a quantitative cut-off of 480 U/L was reported (16). No validated cut-off of PD-L1 expression levels over the threshold of 50% has been formally explored. In a series of 187 patients with high PD-L1 NSCLCs treated with first-line pembrolizumab, patients’ clinical outcomes were significantly improved in very high PD-L1 ≥90% positive tumours (17). Thus, our ROC analyses suggested that in patients with NSCLC treated with an ICI, 5 is an appropriate NLR cut-off for patients with aNSCLC, a quantitative LDH cut-off of 269 could be considered as an alternative to the ULN and a lower threshold of 80% PD-L1 NSCLC expression could be considered. Furthermore, our combined analyses differed from the LIPI (8), besides the use of ROC-based and quantitative cut-offs, for the inclusion of PD-L1 expression level, which currently represents the only validated predictive biomarker.
Regarding the association with disease response, only PD-L1 ≥80% was associated with both RR and PD, while NLR <5 and nLDH with the only PD. Intriguingly, although high PD-L1 seemed to be associated with RR independently of NLR, a nonsignificant trend toward better OS in patients with low NLR and high PD-L1 as compared to those with high NLR and high PD-L1 was observed. This suggests further investigations in comparative trials to explore the predictive value of PD-L1 >80% against the prognostic value of NLR and LDH.
One limitation of this study is the lack of a control cohort. This does not allow the discrimination between the prognostic and predictive value of the factors we investigated. Ideally, the proper control cohort to explore the predictive value of our factors in this specific setting would be the cohort of patients treated with chemotherapy plus the ICI, which currently is unlikely in the clinical practice for patients with high PD-L1 tumours. We are, therefore, conscious that the output of our analysis was to explore an easy-to-assess lab prognostic tool, which could currently aid the decision-making about the addition of chemotherapy to ICI in patients with high PD-L1 tumours, alongside other clinical factors, while waiting for evidence from randomized clinical trials in this setting and the possible exploration of their predictive value. Identifying patients who are likely to benefit from pembrolizumab monotherapy thanks to low NLR and high PD-L1 or nLDH (approximately one third) would be highly relevant to us. The opposite could be even more interesting. Patients with a high PD-L1 (>80%), but NLR >5 and/or high LDH may warrant a combination strategy to offer them the best outcomes. We advocate the clinical evaluation of this easy-to-assess tool based on the combination of low NLR <5 and high PD-L1 >80% or nLDH <269 for retrospective and prospective analyses in clinical trials, comparing the addition of chemotherapy or a different ICI to single-agent immunotherapy. If the validity of this simple tool were confirmed, it could be of crucial importance in the current therapeutic landscape.
Another possible limitation of our study, other than the lack of a validation set, could be the relatively short follow-up time. However, currently available evidence and approval of ICIs in the first-line treatment of NSCLC are based on a median follow-up time from the 12 available studies of 13.0 months, ranging from 7.7 to 25.2. Particularly, the 4 studies with pembrolizumab in the first-line setting had median follow-up of 11.7 months, ranging from 7.7 to 25.2 (1,3).
As above mentioned, since the present study did not compare immunotherapy to chemotherapy plus ICI, we cannot support the predictive value of PD-L1 >80%, or of low NLR <5, but we can suggest their prognostic value, alongside with that of nLDH.
In conclusion, we suggest that low NLR <5, when combined with high PD-L1 >80% or nLDH <269 represents an easily assessable and affordable tool to explore the outcomes of aNSCLC patients with high PD-L1 likely to benefit from pembrolizumab monotherapy. This tool could have a role in therapeutic decision-making alongside other clinical factors. Its validation in retrospective and prospective randomised trials is warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK Reporting Checklist. Available at http://dx.doi.org/10.21037/tlcr-19-583
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-19-583). MB reports personal fees from Janssen-Cilag, Boehringer Ingelheim, Roche, non-financial support from Bristol-Myers Squibb, AstraZeneca/MedImmune, Pierre Fabre, Ipsen, outside the submitted work. DS reports personal fees and non-financial support from Astra Zeneca, Bristol Myers Squibb, personal fees from Lilly, non-financial support from Merck Sharp & Dohme, Roche outside the submitted work. Dr. Metro reports personal fees from Boehringher-Ingelheim outside the submitted work and serves as an unpaid editorial board member of Translational Lung Cancer Research from Jul 2019 to Jul 2021. ADT reports other from MSD outside the submitted work. AF reports personal fees from Roche, Pfizer, Astellas and Bristol-Myers Squibb outside the submitted work. MCG reports personal fees and other from Eli Lilly, personal fees from Boehringer Ingelheim, Otsuka Pharma, grants, personal fees and other from Astra Zeneca, Novartis, BMS, Roche, Pfizer, Celgene, personal fees from Incyte, Inivata, Takeda, grants and other from Tiziana Sciences, Clovis, Merck Serono, grants and personal fees from Bayer, grants, personal fees and other from MSD, grants and other from GlaxoSmithKline S.p.A., personal fees from Sanofi-Aventis, grants and other from Spectrum Pharmaceutcials, Blueprint Medicine, personal fees from Seattle Genetics, Daiichi Sankyo outside the submitted work. AA reports personal fees from BMS, Astrazeneca, Roche, Pfizer, MSD, Boehringer outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The analysis followed the principles outlined in the Declaration of Helsinki (as revised in 2013) for all human or animal experimental investigations. The analysis involved a real-world series of patients treated according to the clinical practice; ethical approval was waived because all patients in each Center signed a specific consent form for their data collection and sharing with other Institutions.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banna GL, Passiglia F, Colonese F, et al. Immune-checkpoint inhibitors in non-small cell lung cancer: A tool to improve patients' selection. Crit Rev Oncol Hematol 2018;129:27-39. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Addeo A, Banna GL, Metro G, et al. Chemotherapy in Combination With Immune Checkpoint Inhibitors for the First-Line Treatment of Patients With Advanced Non-small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Front Oncol 2019;9:264. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Addeo A, Banna GL, Weiss GJ. Tumor Mutation Burden-From Hopes to Doubts. JAMA Oncol 2019;5:934-5. [Crossref] [PubMed]
- Banna GL, Olivier T, Rundo F, et al. The Promise of Digital Biopsy for the Prediction of Tumor Molecular Features and Clinical Outcomes Associated With Immunotherapy. Front Med (Lausanne) 2019;6:172. [Crossref] [PubMed]
- Tavakkoli M, Wilkins CR, Mones JV, et al. A Novel Paradigm Between Leukocytosis, G-CSF Secretion, Neutrophil-to-Lymphocyte Ratio, Myeloid-Derived Suppressor Cells, and Prognosis in Non-small Cell Lung Cancer. Front Oncol 2019;9:295. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. [Crossref] [PubMed]
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 2005;93:387-91. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019;4:e000457. [Crossref] [PubMed]
- Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 2015;112:1904-10. [Crossref] [PubMed]
- Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016;27:732-8. [Crossref] [PubMed]
- Weide B, Martens A, Hassel JC, et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin Cancer Res 2016;22:5487-96. [Crossref] [PubMed]
- Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014;63:675-83. [Crossref] [PubMed]
- Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol 2019;30:1653-9. [Crossref] [PubMed]