Primary tumor and metastasis—sectioning the different steps of the metastatic cascade
Introduction
Lung cancer is the major cancer worldwide with the highest number of cancer-related deaths. Due to oncogenic drivers detected in non-small cell carcinomas (NSCLC), especially adenocarcinoma, and selective drugs [tyrosine kinase inhibitors (TKIs)] some patients live longer and suffer from less cancer-related symptoms. However, resistance mutations will finally cause recurrence and metastasis, and kill the patient. Immuno-oncology increased the arsenal of oncologic treatments, but even there, resistance does occur, leading to cancer-related death.
Early lung cancer is usually treated by surgery. Even in low stage T1a/b/c and N0/N1, carcinomas recur and will set metastasis within the next 5 years (1). There are several factors responsible for recurrence, such as the status of the immune system, the number of circulating tumor cells (CTCs), the differentiation of the tumor, genetic drivers and cooperating genes, and many more factors, not all yet known.
Patients usually do not die because of their primary tumor, but due to metastatic disease. In this review we will focus on mechanisms of metastasis, the difference between primary tumor and their metastases, and on methods to better predict metastasis, or find targets to inhibit metastasis.
What are the factors relevant for metastasis?
General remarks
First of all, there is a need for clarification, what metastasis is. Many manuscripts on metastasis analyze one aspect, and claim this as a driver/marker for the whole process of metastasis. Metastasis, however, is a complex process, which can be divided into several steps, which may overlap. These steps are outlined in Table 1; in addition, also relevant molecules involved in directing these processes are added.
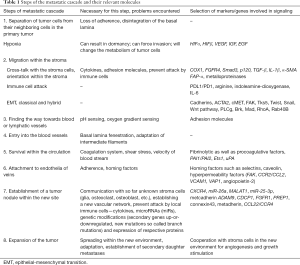
Full table
When different reports are analyzed, it is evident, that these steps are set synonymous for the whole cascade. Factors, which promote migration and/or EMT, are required for metastasis, but tumor cells with this capability might not be able to metastasize to another organ, because they might die within the circulation. Or these tumor cells might be killed by immune cells. On the other hand, migration is also required in the metastatic site, but if the same signaling pathway acts there has not been evaluated. This has to be considered, to better sort the reported data into the metastatic cascade. There is still a lot of work ahead, before we might really inhibit metastasis in patients. So, when reporting and discussing findings on metastasis, it should always be stated, what step of the complex process was investigated.
There are some general phenotypic and genotypic changes tumor cells have to acquire, before being able to metastasize. Tumor cells need to downregulate adherence from either neighboring cells, or need to leave the primary tumor in large complexes [epithelial-mesenchymal transition (EMT), or hybrid EMT]. They need to orient themselves according to the pH and oxygen levels within the stroma (will direct them towards blood vessels or lymphatics) (2-4). If the cells move in complexes, they have to arrange themselves with leading cells and followers (5). The cells need to upregulate some enzymes to degrade matrix proteins, like collagens, express adherence molecules for matrix proteins, and communicate with cells within the stroma (6). In lung cancer most often, the tumor cells communicate with macrophages and induce their polarization into M2 type, which will help to recruit new blood vessels (7). Tumor cells have to protect themselves from attacks by immune cells, either by suppression of killer cells and cytotoxic T cells, expression of PDL1, or accumulation of arginine, to mention a few of the anti-immunogenic programs (7). Finally, tumor cells will enter the circulation, and have to develop mechanisms to avoid being trapped by coagulation (expression and secretion of fibrinolytic enzymes) (8,9), but afterwards will need coagulation to stick to endothelia of venules for leaving the circulation and set up a metastatic focus (10).
The most important process, and still not finally understood is the expansion of the metastatic focus. The microenvironment is ‘new’ to the cancer cell, and the communication has to be established. This is essential for tumor cells, because they need to create a vascular network for growth and expansion. As metastatic clones usually have acquired additional genetic and postgenomic modification, this needs to be evaluated independently from the primary tumor. And even more, in some cases within the metastatic clones there might be a heterogeneity.
Are there genetic differences between primary and metastatic tumors?—clonal heterogeneity
Lung adeno- and squamous cell carcinomas (SCCs) most often present with a dominant trunk mutation(s), which later on is supplemented by branch mutations (11). A trunk mutation is defined as a mutation present in almost all tumor cells, and being kept during evolution of the carcinoma. A branch mutation is defined as a mutation restricted to some carcinoma cell clones; often these are mutations not present in the primary tumor, but in metastases. Driver mutations such as the epidermal growth factor receptor (EGFR) are almost clonal/trunk mutation. Clonal heterogeneity in lung cancer occurs later, especially in metastases. Some common mutations are within the phosphor-inositol-3-kinase CA domain (PI3KCA) and neurofibromatosis type 1 (NF1), in genes associated with chromatin modifications (for example histones), and DNA damage response and repair genes (for example MDM2). Other gene modifications might induce chromosomal instability, copy number alterations, and amplifications. Increased copy number alterations are associated with increased risk for recurrence and death of metastasis. This might be a window, through which the potential of metastasis might be evaluated (see below). Even within pulmonary adenocarcinomas there are different modes of metastasizing with respect to heterogeneity. Everyone reporting lung cancer has seen cases of adenocarcinomas, where despite a small primary tumor (T1) already metastases were present in N2 lymph nodes. On the other end are large tumors (T3), presenting only metastases in N1 nodes and no organ metastasis by radiological investigation. This means there are different modes and timings of metastasizing, independent from the morphological pattern (Figure 1). This directs one to the question, if metastasis does follow a general rule?
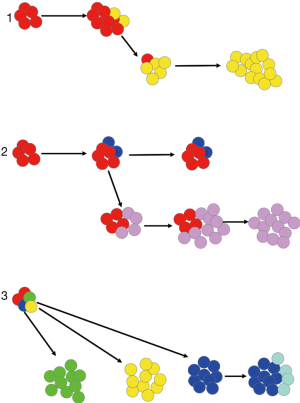
There are extremes known: one clone arises and dominates within a primary tumor, which ultimately will set metastasis. In this case the genetic makeup of the metastases will be very similar with trunk mutations and few additional genetic abnormalities within the metastases. The other extreme is early metastases composed of different clones. These clones might even metastasize at different time and into different organ sites. At the metastatic site these clones might undergo dormancy or expand immediately. Out of these clones re-seeding can occur, i.e., metastasizing into metastases. Within metastases not only genetic modifications can occur, but also protein modifications due to the cross-talk with cells of the microenvironment—i.e., upregulation or downregulation of molecules without any genetic modification (12). In between these extremes different combinations are seen. An example is EGFR-mutated lung cancer. Due to deep sequencing carcinoma cells were identified, which carry the EGFR mutation, and carcinoma cells carrying wild-type (WT) EGFR. Both cell clones are mixed within metastases. When the genetic modifications are compared between metastases and primary tumors an order can be seen in most carcinomas: high similarity score between metastases within the same organ site > lower in metastases in different organ sites > lowest between metastases and primary tumor (12).
The role of hypoxia in tumor cell migration
As the primary tumor grows, the formation of new blood vessels cannot keep with tumor growth, resulting in hypoxia. At this time tumor cells will try to escape hypoxia-induced apoptosis. The most important mechanism is the hypoxia inducible factor 1α (HIF1α): in areas of hypoxia within the tumor, HIF1α is upregulated (13-17). HIFα is translocated into the nucleus where it associates with HIFβ. This dimer induces transcription of vascular endothelial growth factor (VEGF) resulting in increased formation of blood vessels. In addition, growth factors such as insulin growth factor (IGF) and epidermal growth factor (EGF) are also upregulated and inhibit apoptosis (13,18). Carcinoma cells also escape apoptosis and cell death in hypoxic areas by reducing their metabolism and stopping cell division (19), some enter a state called senescence. Tumor cells can switch to an anaerobic metabolism, assisted by autophagy (20), or use an aerobic glycolysis by transformation of pyruvate into lactate regardless of the concentration of oxygen (Warburg effect). Even under increasing concentrations of pyruvate HIF1α is stabilized (21). But hypoxia has another effect for the progression of a carcinoma. Ischemia and hypoxia in lung cancer induce invasion and support migration of carcinoma cells. In KRAS-mutated mouse models of adenocarcinomas necrosis and sometimes hemorrhage preceded the invasion of tumor cells, and invasion was exclusively seen in hypoxic or necrotic areas; similar findings were reported in human pulmonary adenocarcinomas (22). In human tissue samples a release of different proteins related to migration and EMT was proven for hypoxic areas (23-27). If each of these enzymes/proteins act together in concert, or independently is presently unknown.
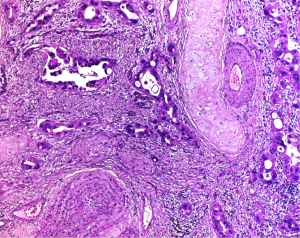
The pulmonary tumor stroma is composed of macrophages, fibroblasts, myofibroblasts, and neutrophils in certain carcinoma types. These cells can either inhibit tumor cell invasion, or cooperate with the tumor cells by remodeling the stroma including the matrix proteins. This can be evaluated by simple morphology: If a classical scar is present, migration of tumor cells is inhibited. The fibroblasts not only deposit different collagens but also elastin, which is known to inhibit tumor cell migration (28). A desmoplastic stroma produced by myofibroblasts is composed of different matrix proteins, and provide an environment, facilitating tumor cell migration (Figure 2) (29,30). Neutrophils are seen especially in SCC. Recently N1 and N2 neutrophils were differentiated (31). Whereas the N1 population often together with M1 macrophages attack tumor cells, N2 neutrophils cooperate with tumor cells. Neutrophils also induce myofibroblast proliferation and differentiation (32). In pleomorphic carcinomas even carcinoma cells undergo an EMT and by this form part of the tumor microenvironment (Figure 3). In rare cases of SCLC besides the classical spheroid cells also an adherent cell population is formed, which might act to provide a tumor friendly microenvironment (33,34).
Myofibroblasts assisting carcinoma cells
Several studies have shown, that tumor associated ‘fibroblasts’ (essentially myofibroblasts in the lung) are different from normal fibroblasts of the lung. They upregulate mismatch repair gene MLH1 and downregulate cyclooxygenase 1 (COX1), fibroblast growth factor receptor 4 (FGFR4), Smad3, and p120 (35). Tumor growth factor β (TGF-β) and interleukin 1β (IL-1β) are driving this differentiation of mesenchymal stem cells into myofibroblasts. TGF-β induces the expression of α-smooth muscle actin (α-SMA) and FAP-α expression, which is essential for movement of the myofibroblasts (36). These myofibroblasts create the matrix structure, which enables the migration of the tumor cells. FAP (serine protease fibroblast activation protein) seems to play an essential role in this respect: FAP depletion increases collagen accumulation, decreases myofibroblasts in number, and blood vessel density in tumors and thus inhibits tumor cell proliferation, whereas FAP expression promotes tumor growth in a K-rasG12D mutant mouse lung cancer model (37). Myofibroblasts also express and secrete matrix metalloproteinases (MMPs) such as MMP-2, MMP-9, MMP-8, and MMP-7. Armed with these enzymes myofibroblasts remodel the extracellular matrix, restructure the collagen network and other proteins, such as fibronectin and tenascin. In addition, MMP-8 plays an active role in myofibroblast migration (38).
The role of matrix proteins
We already have pointed to the importance of matrix proteins for tumor cell migration. Usually the matrix is composed of several proteins such as different types of collagen (I, III, IV, V; in normal lung predominant collagen I), fibronectin, laminin, elastin, and osteonectin. These proteins are cross-linked and provide stability. They also serve as orientation molecules providing ligands for migrating leukocytes expressing adhesion molecules. In NSCLC cells of the tumor stroma selectively synthesize osteonectin (SPARC; normally only in bronchial cartilages) in case of intratumoral hypoxia and acidosis. In areas of hypoxia invasion of tumor cells usually occur, therefore osteonectin can be regarded as an invasion-promoting protein (39).
We already stated that elastin inhibits tumor cell migration in contrast to well oriented collagen deposition. If fibroblasts prevail and unorganized collagens of types I, IV, and V are deposited, this also can inhibit migration. Other matrix proteoglycans such as biglycan, fibromodulin, perlecan and versican also inhibit migration (40). Matrix protein deposition is suppressed by the tumor suppressor gene RNA binding motif protein 5 (RBM5). It is lost in most lung cancer types. RBM5 induces cell cycle arrest and apoptosis, and also decreases cell migration. A loss of RBM5 induces an upregulation of Rac1 (a small GTPase), β-catenin, collagen, and laminin, where Rac1 and β-catenin promote lymph node metastases in lung cancer patients (41). In tumor cells undergoing EMT the cells often express vimentin or fascin. Expression of vimentin is often associated with the expression of periostin in the stroma. This is often seen in more aggressive carcinomas. Together with periostin, versican is often found in advanced tumors, but the function of the latter is not well known (42).
Escaping immune cell attack
During early carcinogenesis tumor cells are recognized by the immune system and destroyed by cytotoxic lymphocytes and natural killer cells. However, this depends on the amount and also the immunogenicity of neoantigens expressed by the tumor cells. But tumors have developed escape mechanisms. Here only a few mechanisms are discussed—a more detailed discussion can be found in another article in this issue.
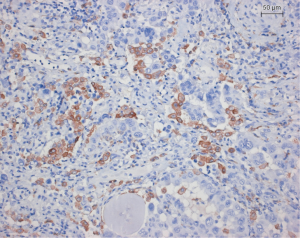
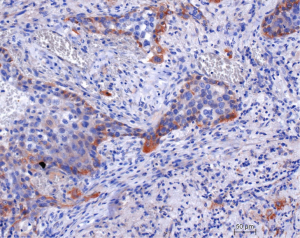
In lung cancer macrophages are seen in most cases. However, macrophages are seen outside the tumor as well as between tumor cells. Macrophages within the tumor are preferentially of M2 type (Figure 4). M2 macrophages in contrast to M1 type are cooperating with the tumor cells. They release many protumorigenic cytokines such as interleukin 8 to assist tumor cells in metabolism and angiogenesis (43). Antigen presenting cells play another major role in tumor cell defense. However, only conventional dendritic cells (DCs) will support tumor cell killing, whereas plasmocytoid and monocytoid DC will protect tumor cells. Tumor cells have hijacked this system: SCLC use bombesin/gastrin-releasing peptide as an autocrine loop for the stimulation of growth. As a side effect, this hormone inhibits the production of IL-12 by DC and this in turn blocks the activation of T cells (44). Plasmocytoid DCs characterized by CD11c(low)CD11b(high) express IL-10, VEGF, and arginase I and inhibit CD4+ T cell proliferation (45). Another cell type, regulatory T cells (Treg) induce immune tolerance for tumor cells: Treg downregulate cytotoxic T-cells and NK cells (Figure 5) (46-48). Another cell population, myeloid-derived suppressor cells (MDSCs), is derived from the bone marrow (BM) (Figure 6). These cells release arginase I, which in turn downregulate a cytotoxic T-cell reaction (49). Indoleamine 2,3-dioxygenase (IDO) and IL-6 probably regulate MDSC, because IDO as well as IL-6 recovered MDSC function and increased metastatic spread. If IDO was knocked down, vascular density was significantly reduced in an experimental model (50).
Regulation of Migration and EMT
Tumor cells migrate either as single cells or small cell clusters, or they move as large clusters of organized cells. The first type of movement is seen in small cell carcinoma, sarcomatoid carcinomas, and in many undifferentiated NSCLC. Well differentiated adeno- and SCCs, such acinar adenocarcinoma and keratinizing SCC prefer a movement in large cell complexes. Migration of single cell and small clusters is probably easier for carcinomas, as single cells can easier change to a spindle cell morphology, which enables better movement between the matrix proteins. There is a lot of changes in tumor cells during migration. To adapt to migration the more rigid intermediate cytokeratin skeleton is exchanged for vimentin, α-actin, or fascin, and adherence proteins, such as E-cadherin are exchanged for N-cadherin. This is seen in pleomorphic carcinomas, carcinosarcomas, high-grade squamous cell and solid or micropapillary adenocarcinomas, and SCLC (5,51-53). Pulmonary adenocarcinomas showing a high expression of the α-actin gene ACTA2 had higher metastatic load and worse prognosis. If ACTA2 was downregulated experimentally by siRNAs and shRNAs, migration and invasion was reduced, whereas the proliferation of the tumor cells was unaffected. In this setting ACTA2 seems to cooperate with c-MET and focal adhesion kinase (FAK) (54). Migration within the stroma requires several changes in tumor cells, the most important function is the formation of invadipodia. Tyrosine kinase substrate 5 (Tks5) is a scaffolding protein necessary for the formation of invadipodia (Figure 7). Short isoforms are associated with reduced, long isoforms with increased metastasis (55,56).
The neural Wiskott-Aldrich syndrome protein (N-WASP) is the regulator for the expression of Tks5 as well as that of α-actin. In addition also the expression of the membrane type 1 MMP-14 (55) is regulated by that gene, which is often affected in pulmonary carcinomas. This is important, because tumor cells need to either directly digest matrix proteins, or call macrophages for help. However, there is no single migration mechanism for each tumor type: Myosin heavy chain 9 (MYH9) and Copine III (CPNE3) when tested in lung cancer cell lines positively correlated with invasion and migration in a transwell system. A knock-down of CPNE3 inhibited metastasis in a mouse model, and in human samples of lung carcinomas a high expression of CPNE3 protein was seen only in higher stages (57). Also, nestin expression correlated positively with tumor size and number of metastases. If nestin was inhibited by shRNA it resulted in reduced proliferation, migration, and sphere formation in adenocarcinoma cells (58).
EMT
EMT is the best studied mechanism of tumor cell migration. In the classical form it results in single cell or small cluster migration type (Figure 8). Genes associated with EMT were most often studied in experimental settings. Twist, Snail, and TGF-β1 were reported in most experiments, performed in cell cultures or experimental models. Twist suppresses E-cadherin expression, which results in loss of adhesion, enhanced motility, and switch to a mesenchymal phenotype—so Twist might be a master regulator of EMT (59). If Twist is inhibited by siRNA in EGFR mutated pulmonary adenocarcinoma cell lines motility and migration was also downregulated (60). TGF-β1, which is upstream of Twist could induce a fibroblast-like phenotype in epithelial cell lines in the study by Pirozzi et al. An inhibition of TGF-β1 elucidated many more downstream downregulated genes, such as Slug, Twist and α-Cat, whereas others were upregulated, such as cytokeratin, E-cadherin, and CD326.
Here some caution for the interpretation of these observations is necessary: Carcinoma cells in general acquire a spindle cell mesenchymal phenotype in 2D cell cultures without any treatment; they also initially can lose cytokeratin and E-cadherin expression, but later on before confluence again express these proteins and stabilize their connection to the neighboring cells. Therefore, expression of EMT markers might be a dynamic process, and when studied in cell culture should include different time points. Interestingly some stem cell markers, such as Oct4, Nanog, Sox2 and CD133, are coexpressed in these EMT experiments, pointing to the involvement of tumor stem cells in this process (61).
Adhesion molecules play an important role in EMT, and since these are regulated by members of the Wnt pathway, Wnt, frizzled, disheveled, catenin, and glycogen synthase kinase 3β (GSK3β) have extensively been investigated. Suppressor of AP-1 (SARI, also called BATF2) modulates GSK3β-β-catenin signaling pathway (62). It upregulates E-cadherin, represses vimentin in pulmonary adenocarcinoma cell lines. If knocked out experimentally in a xenograft mouse model, multiple lymph node metastases arises, and EMT was initiated. In the study by Blaukovitsch upregulation of c-Jun and consecutive overexpression of vimentin and fascin also induced EMT in pulmonary sarcomatoid carcinomas (53), showing that other alternative pathways without Snail or Twist do exist.
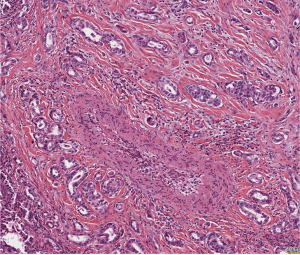
But there exists another migration type: migration in large cell clusters. This is especially seen in well-differentiated pulmonary NSCLC. Adenocarcinomas present with well-structured acini or papillae, SCCs move in large sheets of cells, and these cell clusters can be seen deep within the stroma and within blood vessels (Figure 9). How tumor cells organize this coordinated movement and retain their epithelial structure is almost unknown. In Drosophila a similar migration was described for border cells forming the wings. Four genes were identified as essential for border cell migration: Rack1 (receptor of activated C kinase), brk (brinker), mad (mother against dpp), and sax (saxophone). Rack1 may be important for border cell migration and cluster cohesion maintenance by inhibiting the cytosolic kinase Src (5,63). Although this study was investigated in a pathway analysis of organogenesis, the data provided a first insight into mechanisms of cell complex migration. In the study by Zacharias et al. Twist, phospholipase Cγ (PLCγ), Brk, Mad, Tks5, RHOA (a small GTPase), and Rab40B were identified in bulk or hybrid-EMT migration in pulmonary adeno- and SCCs in over 70% of cases; in contrast Slug, ZEB1, Snail, and TGF-β were not expressed pointing to the differences in signaling pathways between classical and hybrid EMT (5). Bulk migration might provide an advantage for carcinomas: together they might better protect themselves of immune cell attacks by manipulating these cells, test ways of keeping up with hypoxia and nutrient shortage. They also can associate easier with other cells such as platelets, which will assist tumor cells in handling coagulation (64,65). And arriving at a metastatic site bulks of tumor cells might be better prepared for establishing the metastatic focus.
Recently another type of migration was described, amoeboid migration, which is commonly seen in SCLC. In contrast to EMT it is independent from proteolytic degradation of matrix proteins, but require enhanced contractility, usually provided by an increased activation of RHOA (64). These carcinoma cells do not need adhesion molecules, and CTCs derived from these carcinomas earlier form metastatic nodules and progress. Here RHOA expression connect this migration pathway to hybrid EMT.
Vascular invasion, lymphatic/hematologic
Blood vessels—shear stress, coagulation
What are the mechanisms directing tumor cells towards blood vessels? Matrix protein in the tumor microenvironment provide ligands or receptors for adhesion molecules (cytokines), thus helping in orientation of tumor cells. Towards blood vessels a higher oxygen tension, and close to the blood vessels a neutral pH is found. This indicate that tumor cells have sensors for pH and oxygen (8,66). Invasion into blood vessels is similar to stroma invasion, as similar matrix proteins form the vascular stroma (collagen IV, fibronectin, etc.). This type of protein degradation can be facilitated by tumor cells using MMPs. But new challenges are found within the circulation: shear stress due to tumor cell deformation in small blood vessels and the problem of coagulation. Tumor cells expressing vimentin and/or α-actin can adapt to the capillary diameters, whereas cells expressing cytokeratin might burst: especially high-molecular cytokeratins confer a rigid cytoplasmic structure inhibiting adaptation of cell shape, whereas vimentin is much more flexible, and therefore cells can better change the cellular configuration and adapt to capillary diameters. This might be one reasons why a majority of tumor cells do not survive within the circulation (67). However, one aspect has never been explored: there are different types of cytokeratins’ and the association of the acidic and basic cytokeratins’ into intermediate filaments is a dynamic process; tumor cells might change the cytokeratin composition, can dissolve the filaments and reconstitute them according to their need for adaptation within the blood stream. Probably low-molecular weight cytokeratins’, often expressed in carcinomas might be more flexible. Another aspect is the velocity of the blood flow: a recent review by Follain et al. showed, that low shear forces, such as those in small veins will not result in cancer cell disruption; in addition the association with platelets or neutrophils will also add in cancer cell survival (68).
But what about those carcinomas moving as large clusters? From studies investigating CTCs, it is known that the prognosis is worse, if large carcinoma clusters are seen by CTC analysis (69-71). There exist a possible explanation: Tumor cells cannot only migrate in complexes, but also can form complexes with tumor-associated myofibroblasts, neutrophils, and MDSCs (72,73). This association seems to act like a protection shield for the tumor cells. Even within circulation, tumor cells have been found to associate with platelets and neutrophils, and these cells could assist in survival of tumor cell complexes (9,74-76). These cells might help tumor cells to avoid being trapped by coagulation; in addition, within veins these cells might provide coagulation and adherence to endothelial cells, resulting in reducing the speed of blood flow, facilitating the roll-over on endothelial cells and finally extravasate from the circulation (9,68,75). Tissue factors being produced and released by macrophages might assist here. Although neutrophils might also release tissue factors, macrophages seem to be necessary, because impairment of macrophage function decreased tumor cell survival without altering clot formation (77). Mucinous adenocarcinomas have developed another mechanism of clot formation: Mucins induce clot formation, platelet aggregation, and interact with L-selectin and platelet-derived P-selectin without thrombin generation (78).
Coming back to tumor cell trapping by clots: macrophages and granulocytes can assist carcinoma cells activating fibrinolysis. The classical fibrinolytic components as tissue plasminogen activators (t-PA), and the inhibitors PAI-1 and PAI-2 were not expressed in pulmonary carcinomas, whereas urokinase-specific antibodies stained tumor cells and macrophages and PAI-1 and PAI-2 were expressed in interstitial and alveolar macrophages (79). In another study pulmonary adenocarcinomas expressed ETS domain family of transcription factor 1 (Ets-1) and urokinase-type plasminogen activator (u-PA) (80). So different types of handling with the coagulation system do exist in different carcinomas.
Lymphatic vessels
Invasion into lymph vessels is easier for tumor cells due to the thin wall. Carcinoma cells spread via interstitial channels into the lymphatic vessels, but tumor cells can also congest lymph vessels. This might reverse the lymph flow, and explain unusual sites of lymph node metastasis, such as metastases in abdominal nodes. However, within the lymphatic system tumor cells have to deal with the immune system. Immune escape mechanisms will play an important role, and propagation of tumor cells might be protracted for a while. This might be a reason, why metastasizing via the lymphatic system will result in later distant metastases compared to hematologic spread (81). However, it should be noted, that tumor cells can enter blood vessels present within lymph nodes.
Extravasation of tumor cells—coagulation, hyperpermeability, selectins
After having discussed the function of platelets and coagulation for the carcinoma cells to facilitate extravasation, several other questions arise: extravasation seems to be site specific. Homing factors most likely play an important role, similar to what is known from homing of lymphocytes. The most important sites are venules with high endothelia. Adhesion molecule receptors and their ligands are expressed on tumor cells and on endothelia. By coagulation or simple aggregation of platelets on endothelia the blood flow is reduced, enabling tumor cells to roll over the endothelia and adherence can occur. Hyperpermeability is another factor, which slows down the blood flow. Microthrombi enable a firm adherence of the tumor cells to the endothelia and by different enzymes holes are produced between endothelial cells to facilitate migration of tumor cells into the new organ site. Caveolin seems to be one of the factors associated with vascular permeability, as loss results in increased permeability and tumor growth (82). Hyperpermeability is also mediated by endothelial cell FAK, which up-regulates E-selectin, leading to preferential homing of metastatic cancer cells to these foci (83). Another factor is MD-2 upregulating chemokine receptor type 2 (CCR2), which together with its ligand CCL2 induces the release of serum amyloid A3 and S100A8, factors inducing hyperpermeability (84). Tumor cells once attached to endothelia induce the formation of vascular cell adhesion molecule-1 (VCAM-1) and vascular adhesion protein-1 (VAP-1). These two factors cause clots and also activation of tissue coagulation factors (85,86). Between endothelial cells cell-to-cell junctions are formed; these junctions are dynamic, which means interendothelial gaps are formed. Angiopoietin-2 can induce such gaps, which tumor cells use for migrations through the vessel wall (87).
Tumor cells use different selectins such as E- and P-selectin to adhere to specific sites on the endothelia of venules. Other selectins have been shown to be active by knockdown experiments: PSGL-1, CD44, and CEA were detected in SCLC cells. By intravital microscopy SCLC cells were shown to roll along vessel walls mimicking leukocyte behavior (88). Recently mechanisms were elucidated, how the microenvironment is manipulated and veins with high-endothelia are formed already before tumor cells have entered. VEGF growth factors play an important role in this process, whereas the antagonist bone morphogenic protein 4 is downregulated (89-91). Here again inflammation plays an important role, as it increases the expression of E-selectin, one of the homing factors for tumor cells. In SCCs other molecules act in facilitating metastasizing: high levels of VLA-1 and VLA-2 two members of the β1 integrin family increased the number of metastases (92).
Preparing the distant metastatic focus
We already discussed that only few tumor cells survive within the circulation. But moreover, only a small fraction of tumor cells, reaching a metastatic site progress and form a metastatic focus (93). Studies focusing on CTCs have shown, that single tumor cells usually do not form metastatic micronodules, whereas large clusters confer a significant risk of metastasis (94). A few carcinoma types such as SCLC are an exception, because small cell carcinoma cells are usually numerous within the circulation (95,96).
Tumor cells prefer different organs, for example SCLC and adenocarcinomas (especially those with EGFR mutations or AKL rearrangements) preferentially set brain metastases, whereas SCCs prefer bones (97-99). How this selection is facilitated? In general, tumor cells have to communicate with cells in their new microenvironment. In the brain carcinoma cells need to cooperate with astrocytes for angiogenesis, and manipulate microglia cells and macrophages to avoid attacks. These aspects of metastasis are still not fully understood, and need further research. Homing factors most likely play a major role in the selection of metastatic sites, but there are still many factors not well known in lung cancer.
The group by Sadanandam identified 11 unique peptides, specific for homing to lung, liver, BM, or brain. Semaphorin 5A and its receptor Plexin B3 were identified as relevant for homing to these organ sites (100). Another factor for homing is the chemokine receptor CXCR4. But CXCR4 is not site selective, as its expression was found in metastases within colon, lung and breast (101). CXCR4 seems to be required for a communication with stroma cells (Figure 10), which subsequently help to prepare a metastatic “niche” (102,103). This view is substantiated by the finding, that CXCR4 can be found traveling within exosomes, being released in bone, and induce an activation of osteoclasts. These cells prepare a metastatic niche long before carcinoma cells enter the bone (104). Another function of CXCR4 is the regulation of MDSCs: inhibiting CXCR4 also inhibits the influx of myeloid-derived cells and subsequently reduces the number of metastases (105). Also micro-RNAs (miR) influence metastasizing: Liu et al. found that expression of miR-26a dramatically enhanced lung cancer cell migration and invasion (106). They showed, that miR-26a positively regulated MMP-2, vascular endothelial growth factor (VEGF), Twist and β-catenin, whereas phosphatase and tensin homolog (PTEN) was negatively regulated. Thereby miR-26a enhanced the process of lung cancer metastasis by suppressing PTEN, resulting in activation of the AKT pathway. In addition, miR-26a increased AKT phosphorylation and nuclear factor kappa B (NF-κB) activation, which act on growth and metabolic adaptation.
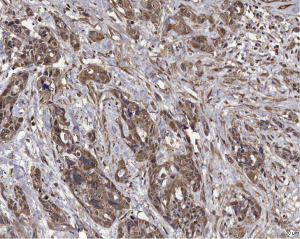
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) seems to be another regulator of metastatic focus formation: deficient cells are impaired in migration and form fewer tumor nodules in a mouse xenograft. Expression of MALAT1 enhances lung cancer metastasizing (107). The function of MALAT1 is linked to an upregulation of eIF4A1 and thymosin β4 in promoting metastases in NSCLC (108).
Analyzing CTCs of breast cancer, some clones activated the androgen receptor, and selectively established bone metastases, whereas other clones activated Notch, TNFα, IL-1β, NF-κB, CXCL8, CXCR4, and PDGBB, and these clones established brain metastases (109,110). It is very likely, that similar mechanisms do exist in many other cancer types such as lung cancer, as these proteins point to a selective communication with the microenvironment at the metastatic site.
Exosomes preparing the metastatic microenvironment
Exosomes are small vesicles released from cells including tumor cells. They facilitate communication between cells. Packed in these vesicles are proteins and lipoproteins, RNAs including miRs. Some of these vesicles act between neighbor cells—in tumors they might transmit information how to counteract against chemotherapeutic drugs. More important are exosomes, which circulate in body fluids and might contain information, how to prepare a metastatic niche, long before tumor cells enter these sites. Exosomes secreted from tumor cells are called tumor-derived exosomes (TDE). TDE can construct an environment supporting tumor proliferation, angiogenesis, invasion and premetastatic niche preparation. TDE may inhibit the immune system and thus protect tumor cells. TDE might also confer chemoresistance via removal of chemotherapeutic drugs (111). Exosomes can influence every step of the metastatic cascade and might be targeted by oncological treatment (112). TDE can influence coagulation, vascular leakiness, and reprogramming of stromal recipient cells to support premetastatic niche formation and subsequent metastasis (113). Within exosomes mRNA, miR, and proteins can be detected. They can serve as messengers and carriers in lung carcinogenesis. TDE play a role in lung cancer by influencing EMT, oncogenic cell transformation, angiogenesis, metastasis and immune response in tumor microenvironment (114). The role of exosomes in cross talk with cells in the microenvironment of the premetastatic niches is being explored and so far, it seems that TDE play a major role in extravasation of tumor cells and the seeding efficacy of tumor cells within their metastatic sites, and it seems to be selective too. Cell migration-inducing and hyaluronan-binding protein (CEMIP) is elevated in exosomes from brain metastatic, but not lung or bone metastatic cells. CEMIP depletion in tumor cells impaired metastasizing to the brain, disrupts invasion and tumor cell association with the brain endothelial cells. Uptake of CEMIP-containing exosomes by brain endothelial and microglial cells results in endothelial cell branching and inflammation by expression of pro-inflammatory cytokines, such as Ptgs2, TNF and CCL/CXCL (115). In another experiment exosomal miR-25-3p, a metastasis-promoting miRNA of colorectal cancer, can be transferred to endothelial cells, regulating the expression of VEGFR2, ZO-1, occludin and Claudin5 in endothelial cells. This again results in vascular permeability and angiogenesis. In addition, exosomal miR-25-3p induces vascular leakiness and enhances metastasizing to liver and lung in mice (116). In addition to selectins, which we discussed above, also exosomes are involved in the selection of metastatic sites. In experimental breast carcinomas the metastatic versus the non-metastatic carcinomas differ in their exosomal contents: metastatic exosomes contain a distinct set of membrane proteins including ceruloplasmin and metcadherin, which could presumably aid in directing cancer cells to specific metastatic sites (117). Stroma cells play a prominent role in invasiveness and metastatic potential of different carcinomas. Also, in this aspect TDE seem to play a role. Exosomes released by bladder cancer cells are internalized by fibroblasts and promoted proliferation. Cancer cell-derived exosomes contain TGF-β and activate SMAD-dependent signaling in fibroblasts. Therefore, bladder cancer cells trigger the differentiation of fibroblasts to tumor-associated fibroblasts by exosomes-mediated TGF-β transfer and SMAD pathway activation (118). Investigations on TDE in pulmonary carcinomas is still in its infancy, but similar mechanisms can be expected in the future. The analysis of exosomes is still not a routine procedure, as it requires ultracentrifugation, separation of the exosomes, before their content can be analyzed. However, it can be foreseen, that new technical development will make these procedures easier. Protocols for the separation of DNA, different kinds of RNA, and proteins are already available and might be used immediately.
Metastasis—many open questions remain
Some questions are open, some might be answered in this paragraph, but more will need further investigations:
- Do tumor cells leave the primary tumor early or only when a certain size is reached?
- Within a primary tumor different clones can arise—can they all set metastasis or only certain clones?
SCLC usually migrate in small clusters or single cells (amoeboid or EMT type). When these carcinomas are evaluated for their microenvironment there is often minimal stroma reaction, and a small primary tumor, undetectable by CT scan has set large cerebral metastases. This points to early metastasis. On the other extreme, SCCs can reach a size of several centimeters, but has not set many distant metastases. In adenocarcinomas both extremes can be encountered: small primary tumor and widespread metastases and large tumors with few metastases—often this can be attributed to special types, such as acinar versus micropapillary or mucinous adenocarcinomas (Figure 11). This might be due to genetic heterogeneity, which can exist not only among different pulmonary adenocarcinomas, but also heterogeneity even among different metastases arising from one primary tumor (11). Generally pulmonary carcinomas show preferences for metastatic sites: bone 34.3%, lung 32.1%, brain 28.4%, adrenals 16.7%, and liver 13.4% (97,102). EGFR mutated and ALK rearranged adenocarcinomas have been accused to present with increasing frequency of cerebral metastasis. But Hendriks et al. reported that in their cohort of 189 patients there was no difference between EGFR-, KRAS-mutated, or WT adenocarcinomas. There was only a longer post metastatic bone disease survival in EGFR-mutated patients (119). In contrast to that Shin et al. observed a strong association between EGFR mutation status and brain metastases, whereas no association was found between EGFR mutation status and extracranial metastases. In addition, the number of brain metastases was significantly correlated with the EGFR mutation status (120). Riihimäki et al. reported increased cerebral metastasis in women and younger patients, which would fit to the study above, as in this population the percentage of EGFR mutations is increased (97). All these reports point to genetic heterogeneity: very likely clonal evolution and acquiration of branch mutations play a role; acquiring secondary branch mutations might provide fitness to the tumor cells in communicating with the stroma of the metastatic sites and expansion of the carcinoma. KRAS mutation in experimental pulmonary adenocarcinomas alone do not result in metastasis, but secondary mutations in genes such as TP53, STK11, or CDKN2A, will cause metastasis outside the lung (121,122).
The clonal evolution has been discussed, but with respect to their metastatic potential the data are scarce in pulmonary carcinomas. New information might be expected from ongoing studies.
Common and most important sites of metastases—brain, lung, bone
Finally, we will focus on three metastatic sites of lung cancer, for which data have been reported, and which are the most important ones for clinical management.
Metastasis to the brain
Cerebral metastasis is one of the major life-limiting factors in lung cancer. Pulmonary carcinomas colonize the brain in different ways: Small cell carcinoma diffusely infiltrate the brain, whereas adenocarcinoma most often form circumscribed nodules. This has nicely been demonstrated by a coculture system consisting of an organotypic mouse brain slice and 3D cell spheres of epithelial cells embedded in Matrigel (123). Cancer cells are attacked by macrophages and lymphocytes in the early stage, whereas microglia cells do not cooperate (124). In adenocarcinomas often a pseudocapsule is formed, which inhibit carcinoma cell spreading for some time—this capsule help in defining the resection margins during surgery. Microglia support colonization of brain tissue (Figure 12), probably because these cells have been manipulated by the upregulation of the Wnt pathway. If an inhibitor of Wnt, Dickkopf-2 is upregulated, the prometastatic action of the microglia is impaired (123). This view was confirmed by another study showing an upregulation of the Wnt-pathway members Dishevelled-1, Dishevelled-3, E-cadherin, and β-catenin in metastatic foci of SCLC and pulmonary adenocarcinomas (125). Independently of β-catenin, lymphoid enhancer-binding factor 1 (LEF1/TCF4) also promoted cerebral metastases in human lung adenocarcinomas by downregulating the expression of E-cadherin (125,126). So far, we have predominantly discussed studies on SCLC and adenocarcinomas, but also in SCCs the similar mechanisms are acting: An increase of β-catenin, E-cadherin, and decrease of CD44v6 and caspase-9 expression in peritumoral brain edema was shown in metastases of SCC (127,128). Similarly, an increase of caspase-9, CD44v6, and decreased cellular apoptosis susceptibility (CAS) protein and Ki-67 was found in SCC cerebral metastases (128). How many members of the adherence protein family are involved in metastasis was nicely shown by the study of Nasser, showing an inverse correlation of E-cadherin expression and development of brain metastases (129). In a mouse model a treatment with pioglitazone, a peroxisome proliferator-activated receptor γ-activating drug prevented loss of E-cadherin expression. This resulted in inhibition of the expression of MMP9 and fibronectin, and furthermore the development of brain metastases.
Astrocytes too cooperate with tumor cells. Astrocytes can release MMP-2 and MMP-9, which assist in tumor cell invasion (130). In this setting the coagulation cascade plays an important role: cancer cells prevent plasmin generation by neuroserpin and serpin B2 (131). Therefore, the conversion of membrane-bound FasL on astrocytes, a death signal for cancer cells is inhibited. Furthermore, the inactivation of the adhesion molecule L1CAM, which supports cancer cell movement along brain capillaries is also inhibited.
ADAM metalloproteinase 9 (ADAM9) levels were relatively higher in brain metastases compared to levels in primary lung tumors. ADAM9 is a member of the disintegrin and metalloprotease domain family. These are membrane-anchored proteins involved in cell-cell and cell-matrix interactions. In lung cancer ADAM9 induces plasminogen activator-mediated cleavage of cub-domain containing protein 1 (CDCP1), a promigratory protein. This is promoted by Src/Abl kinase, because an inhibition of this kinase by dasatinib inhibited migration of cancer cells (132). ADAM9 also regulates miR-218: if ADAM9 was inhibited, SLIT2 a secretory protein and miR-218 were upregulated; both induced downregulation of cadherin-2 (CDH2). This means that ADAM9 activates CDH2 by an inhibition of miR-218 in lung adenocarcinoma (133). ADAM9 also induces ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor.
Are there differences between oncogene-driver induced pulmonary adenocarcinomas and those induced by cigarette smoking?
In ALK rearranged adenocarcinomas FGFR1 was significantly higher expressed in brain metastases compared to the primary tumor and other visceral metastases (134). A cross-talk of EGFR-MET was reported in adenocarcinomas with brain metastases. This was independent of EGFR mutation and was facilitated via an activation of mitogen-activated protein kinases (MAPK). MET was directly involved in migration and invasion (135). Also, CXCR4 seems to play a role in metastasizing to the brain. CXCR4 protein was highly overexpressed in patients with brain-specific metastasis, but significantly less in NSCLC patients with other organ metastases and without metastases (136,137). So far there seems not to exist a major difference between these two types of adenocarcinomas.
We have discussed the question about clonal heterogeneity above. This is still not solved, because it seems that both possibilities are probably working: clones with additional genome modifications different from the primary tumor metastasize into the brain, and also clones already present in the primary tumor are capable of metastasizing. The study by Mock et al. reported on genes, which were only amplified in the metastatic tumor and are related to leukocyte migration and organ development, pointing to the second option. Genes with a lower copy number in the metastatic tumor were related to proteolysis, negative regulation of cell proliferation and cell adhesion (138,139). On the other hand the analysis of the lung cancer project in the UK have shown, that in pulmonary carcinomas most often trunk mutations are present, and modifications occur later in the metastases, with different gene modifications even within metastases in the same patient (we use the term modifications, because these are not only mutations, but also amplifications, posttranslational modifications, different splice modification, etc.) (11,140,141).
A major problem arises from marker studies, focusing on single genes/proteins. There might be a selection bias due to the selected tissues or just an over interpretation—an example is the long non-coding RNA MALAT1. Shen and coworkers found higher levels of MALAT1 in brain metastases compared to other extrapulmonary sites. In the in-vitro experiments it turned out that the major function of this lnRNA is EMT (142). MALAT1 is required for tumor cell migration and invasion, very likely not only in brain tissue. In another study copy number variations of LKB1 (also STK11) and KRAS mutation predicted metastasizing to the brain (143), however, it is well known, that the combined mutation of KRAS and LKB1 confers a worse prognosis irrespective of the tumor stage and no selection for brain metastases were found (144). In the study by Hwang et al. miR-95-3p was investigated (145). In their model miR-95-3p suppressed tumorigenicity and brain metastases and increased overall survival. miR-95-3p acted by suppressing cyclin D1 and ABLIM2, an actin binding LIM protein family member 2. Another cytoskeleton organizing and motility promoting gene for actinin α4 (ACTN4) was highly expressed in metastatic brain tumors but not in the primary tumor (146). This study nicely points to clonal expansion but cannot be assigned as brain specific, as no other metastatic sites were investigated. In both investigations the focus was on cytoskeletal proteins, which are associated with increased motility, and therefore might not directly act as metastasis genes but rather influence migration.
Similarly, a report discussed pre-B-cell leukemia homeobox (Pbx)-regulating protein-1 (PREP1) as a gene found in cerebral metastases of different solid tumors. Again PREP1, when overexpressed together with TGF-β triggers EMT (147). PREP1 modulates the sensitivity to SMAD3 and induces the expression of Fos-related antigen 1 (FRA-1). Both FRA-1 and PBX1 are required for transition into a mesenchymal phenotype in lung tumor cells. This is another example of interpreting EMT synonymous to metastasis. Han et al. investigated lysine demethylase 5B (KDM5B) and class-3 histone deacetylase (SIRT1) genes, and showed that these genes are involved in motility. If these genes were knocked down, lung cancer cell migration was inhibited. They concluded that SIRT1 correlates with migration in brain metastases (148). But SIRT1 is one of the molecules associated with autophagy and help cells to survive in hypoxia. And hypoxia is common in tumors, and hypoxic areas in mouse models is where invasion takes place. So, the expression of SIRT1 might be related to hypoxia, a common phenomenon in most malignant tumor and not to migration? The discussion of these reports above do not negate the value of these studies, but highlights problems in the interpretation: it would be most helpful, when the major function of an investigated molecule is focused to a step in the metastatic cascade instead of the whole metastatic cascade.
Whereas most studies on brain metastasis focused on lung adenocarcinoma, Paik and coworkers studied SCCs, which less often present with brain metastases. They found that ‘truncal’ PTEN loss and PI3K-aberrations to be associated with brain metastases. There was also a genetic heterogeneity between lung primaries and brain metastases (149).
Many open questions remain: microglia cells and monocytes from the circulation can act against tumor cells, but even monocytes within brain, once differentiated into macrophages can further differentiate into M1 or M2 types. How these events are regulated and at which stage of the development of metastasis this occur is unknown. The normal blood-brain barrier is broken in metastasis, the vessels are leakier, and therefore transgression and invasion take place. These processes are presently in focus of research but answers are not yet available.
Metastasis to the lung
Although pulmonary metastasis is the most common site in all lung carcinomas, not much is known about specific molecular mechanisms. In the study by Elzarrad connexin-43 was identified as adhesion molecule facilitating ‘homing’ to lung endothelial cells. Connexin-43 was highly upregulated in tumor cells during endothelial cell contact (150). In a study of breast cancer cell lines metadherin was identified on carcinoma cells. A lung homing domain of metadherin seems to facilitate binding to endothelia of pulmonary blood vessels (151). This was later on confirmed in an analysis of exosomes derived from breast cancer cell lines: the metastatic clones secreted exosomes, which contained ceruloplasmin and metadherin. So probably metadherin might facilitate homing of carcinoma cells to the lung (117). No data exist for pulmonary carcinomas so far.
Metastasis to the bone
In bone metastasis two different scientific questions are in the main focus: homing mechanisms and colonization, which includes the interaction of the tumor cells with the stroma of bone and BM. In the work of Yang PDGFRβ was found to be the main tyrosine kinase expressed in BM stromal ST-2 and MC3T3-E1 preosteoblastic cells. If ST-2 and human BM endothelial cells are targeted by sunitinib, a PDGFRβ inhibitor, growth is inhibited and apoptosis induced. Sunitinib induced an extensive disruption of tissue architecture and vessel leakage in the BM cavity. Sunitinib pretreatment also blocked the adhesion of lung cancer cells. If mice are pretreated with sunitinib before they were inoculated intracardially with A549M1 or H460M5 cells homing of these adenocarcinoma cells was markedly inhibited. A pretreatment of the tumor cells however, had no effect (152).
Osteoclasts are important cells for remodeling of the bone under normal condition, but also in metastasis. These cells can create a metastatic “niche”, long before tumor cells invade. Knockdown of discoidin domain receptor 1 (DDR1, a gene mutated in SCC) by siRNA showed reduced invasiveness into collagen matrices and increased apoptosis. This effect of decreased osteoclast activity could also be induced by a conditioned cell culture medium derived from these cells. A bone metastasis model lacking DDR1, achieved decreased metastatic activity and reduced tumor burden and osteolytic lesions. These resulted also in a substantial reduction of tumor cells reaching the bone compartment (153). Vincent et al. used a lung cancer model in mice. A robust bone colonization was achieved with TGF-β. By transcriptome analysis the authors identified TCF4 and protein kinase D3 (PRKD3) as downstream mediators of bone resorption and enhanced stroma-dependent metalloproteolytic activities. In addition, MCAM and SUSD5, two anchorage-related proteins, enhanced this colonization. Inhibition of TGF-β and metalloproteinases markedly reduced bone tumor burden (154). Tang et al. showed that stromal cell-derived factor-1 (SDF-1) secreted by osteoblasts and BM stromal cells activated the CXCR4/ERK/NF-κB signal transduction pathway, which resulted in increased expression of MMP-9 (103). By analyzing miRs associated with bone metastases of lung adenocarcinomas seven miRs were down and 21 miRs upregulated (155). The MAPK-Wnt-NF-κB signaling pathway was targeted by these miRs, and downstream of this pathway MMPs, cytoskeletal proteins and angiogenesis factors were found to orchestrate bone metastases. Luis-Ravelo investigated the function of RHOB, a small GTPase. If RHOB was silenced by siRNA, metastasizing to the bone was inhibited in a mouse model (156). Interestingly changes in the cellular composition of blood and BM, namely thrombocytosis, but also weight loss, and increased AKP and CEA levels were correlated with bone metastases in patients with pulmonary adenocarcinoma (157). What is lacking in these studies is a functional approach to correlate all these markers and pathways into a stepwise model of bone metastasis: preparation of the metastatic niche, homing of carcinoma cells, cross-talk with the stroma, getting access to blood vessels (nutrition, oxygen), and finally regulating growth at the metastatic site.
Finally, we will discuss one of the promising molecules, which might become a target for bone metastasis inhibition, the RANK-RANK ligand system. This system regulates the activity of osteoclasts. CCL22 upregulated receptor activator of nuclear factor-κB ligand (RANKL) in osteoclast-like cells, induced cell migration, and also enhanced phosphorylation of protein kinase B/Akt and extracellular signal-regulated kinase (ERK). The binding receptor is CCR4, which is often expressed in cancer cells. Therefore CCR4-expressing cancer cells might activate osteoclasts by binding to CCL22 (158). In vivo experiments showed, that a treatment with RANK-antibodies induced lytic lesions and inhibited tumor growth (159). This experimental finding was confirmed by a clinical study: Dougall and coworkers applied Denosumab, a fully humanized monoclonal antibody against RANKL, and demonstrated a prevention or delay of bone metastases in solid tumors, including lung carcinomas. These authors also showed, that RANKL stimulates metastasis, if the cancer cells expressed RANK (160). Peng and colleagues showed upregulated RANKL, RANK, and TNFRSF11B/OPG in NSCLC cell lines and in tumor tissues with bone metastases (161). Treating cell lines with recombinant human RANKL or transfecting them with RANKL-cDNA enhanced migration and invasion. Addition of TNFRSF11B caused inhibition. TNF receptor superfamily member 11b (TNFRSF11B or OPG) codes for an osteoblast-secreted decoy receptor that functions as a negative regulator of bone resorption. Miller et al. confirmed this study, showing that tumor cell-mediated osteolysis occurs through induction of RANKL (162). In a NSCLC bone metastasis mouse model TNFRSF11B-Fc reduced the development and progression of osteolytic lesions. If combined with docetaxel, growth of skeletal metastases was inhibited.
The question how RANKL is regulated was in the focus of the work of Kuo and coworkers. They focused on the parathyroid hormone-related protein (PTHrP). PTHrP and miR-33a regulate RANKL expression, where miR-33a inhibit RANKL as well as macrophage colony-stimulating factor (M-CSF) on osteoblasts. miR-33a downregulated PTHrP, which subsequently also decreased IL-8 secretion. Altogether this resulted in osteoclast differentiation and resorption of bone (163).
All these studies focused on osteolytic bone metastases, but lung cancer metastasizing to bone produce osteolytic as well as osteoblastic lesions (Figure 13). This means there is still a lot to explore.

The evaluation of CTCs in metastasis
The possibility to analyze DNA, RNA, and proteins derived from tumors within the blood (liquid biopsy) opens a new way of studying metastasis in vivo and in patients. The analysis of cell-free DNA (cfDNA) has already entered pathological routine protocols. cfDNA is now used to search for resistance mutation in pulmonary adenocarcinomas. cfDNA can be used to monitor therapy, as under successful therapy the values of a mutated gene drop down and might increase in case or recurrence. For exosomes see the paragraph above.
The analysis of CTCs is already used in some pathological laboratories, and can bring additional information to the patient management. At this moment the number of CTC will allow a prognosis in patients, who underwent surgery for their lung carcinomas: high numbers of CTC predict early recurrence (1,94,164). However, there are limitations. In small tumors often no CTCs can be harvested. CTCs are harvested from peripheral blood, where they are already extensively diluted. Most often this change in large widely metastatic tumors, although even in these sometimes no CTCs are found. Another option is to harvest CTCs during surgery from the draining blood vessels—pulmonary veins in lung cancer. Here the dilution effect is less pronounced. The technology for CTC harvesting and analysis is fast moving and automated systems are now available with many new markers to capture CTCs. So, in the future this will provide more information on individual carcinomas. A clonal evolution of tumors and their metastatic clones can be evaluated, as RNA and DNA can be extracted and analyzed from single cells.
Tumor-educated platelets (TEPs)
Platelets play a role in metastasis, which has been recently reported. These platelets are called TEPs. TEP samples from patients with different tumor types, including lung, brain, and breast cancers, have been tested, and it has been shown that TEPs from patients with cancer are distinct from those with inflammatory and other noncancerous diseases. TEPs can associate with CTCs within the circulation, protect tumor cells from shear and oxygen radical induced stress (68,74,165). TEPs are involved in the progression and spread of several solid tumors, and spliced TEP-RNA surrogate signatures can provide specific information on the presence, location, and molecular characteristics of cancers. So far, it remains to be investigated, how platelets are “educated”, and which mechanisms cause intraplatelet RNA splicing. In an investigation of patients with NSCLC, WASF1, PRKAB2, RSRC1 ribosomal protein module, PDHB carbohydrate-metabolism module, and three other molecules (TPM2, MYL9, and PPP1R12C) may play important roles in NSCLC tumorigenesis and progression. Platelets are also important for adhesion at the metastatic site (74,165,166). This is another important field for research and might provide new therapeutic options.
General aspects for therapy
With the identification of driver mutations, a major step forward was done in treating pulmonary adenocarcinomas. Some hope is on the horizon for a treatment of neuroendocrine tumors. However, our options for preventing metastasizing are still limited. What I have highlighted in the previous paragraphs can be summarized into a statement like: not a single molecule will enable us to interfere with metastasizing. We need to attack tumor cells on several vulnerable structures, such as homing mechanisms (adhesins), inhibit communication with stroma cells within the microenvironment (cytokines), and block angiogenesis and growth factors. Such a combined approach needs to be discovered and tested. There is some hope, that in the future we might be able to block homing and extravasation of metastatic tumor clones by specific molecules, which would be a major step forward in patient management.
Conclusions
In this review metastasizing was dissected into different steps, starting with invasion and migration, invasion into vessels, circulation within the blood vessels, extravasation, formation of a metastatic micronodule/focus, and expansion of this focus. Once tumor cells have invaded the stroma, they need to communicate with stroma cells, orient themselves along matrix proteins, prevent attacks by immune cells. A major problem is survival within the circulation once tumor cells have entered blood vessels. Shear stress and velocity of the blood stream are important factors, which influence the survival of tumor cells. Manipulating the coagulation system is another factor influencing tumor cell survival; some carcinomas use enzymes, expressed in their cytoplasm, other call for assistance by leukocytes and platelets. The next step is finding the right site for adherence and extravasation using homing factors. After facilitating extravasation, the tumor focus needs communication with the stroma in the metastatic site for growth. Tumor vessels have to be induced to provide oxygen and nutrition. Genes play a major role in all these steps. A clonal evolution with secondary gene modification is important for pulmonary carcinomas. These branch mutations very likely make the carcinoma cells fit for these different steps. In addition, posttranslational modification of proteins involved in adherence and migration also play a role. Different other cell types are manipulated by the carcinoma cells to assist in migration, circulation, homing, and expansion of the metastatic focus. Tumor cell derived exosomes and TEPs are other factors important for the preparation of metastatic sites.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Helmut H. Popper) for the series “New Developments in Lung Cancer Diagnosis and Pathological Patient Management Strategies” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-175). The series “New Developments in Lung Cancer Diagnosis and Pathological Patient Management Strategies” was commissioned by the editorial office without any funding or sponsorship. Dr. Helmut Popper served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor of Translational Lung Cancer. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chemi F, Rothwell DG, McGranahan N, et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med 2019;25:1534-9. [Crossref] [PubMed]
- Logozzi M, Spugnini E, Mizzoni D, et al. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev 2019;38:93-101. [Crossref] [PubMed]
- Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Arch Pharm Res 2019;42:833-47. [Crossref] [PubMed]
- Swenson ER. Hypoxia and its acid-base consequences: from mountains to malignancy. Adv Exp Med Biol 2016;903:301-23. [Crossref] [PubMed]
- Zacharias M, Brcic L, Eidenhammer S, et al. Bulk tumour cell migration in lung carcinomas might be more common than epithelial-mesenchymal transition and be differently regulated. BMC Cancer 2018;18:717. [Crossref] [PubMed]
- Gemma A, Takenaka K, Hosoya Y, et al. Altered expression of several genes in highly metastatic subpopulations of a human pulmonary adenocarcinoma cell line. Eur J Cancer 2001;37:1554-61. [Crossref] [PubMed]
- Brcic L, Stanzer S, Krenbek D, et al. Immune cell landscape in therapy-naive squamous cell and adenocarcinomas of the lung. Virchows Arch 2018;472:589-98. [Crossref] [PubMed]
- Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev 2016;35:75-91. [Crossref] [PubMed]
- Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res 2004;64:8613-9. [Crossref] [PubMed]
- Hataji O, Taguchi O, Gabazza EC, et al. Increased circulating levels of thrombin-activatable fibrinolysis inhibitor in lung cancer patients. Am J Hematol 2004;76:214-9. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Hunter KW, Amin R, Deasy S, et al. Genetic insights into the morass of metastatic heterogeneity. Nat Rev Cancer 2018;18:211-23. [Crossref] [PubMed]
- Kothari S, Cizeau J, McMillan-Ward E, et al. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene 2003;22:4734-44. [Crossref] [PubMed]
- Chen YQ, Zhao CL, Li W. Effect of hypoxia-inducible factor-1α on transcription of survivin in non-small cell lung cancer. J Exp Clin Cancer Res 2009;28:29. [Crossref] [PubMed]
- Wan J, Ma J, Mei J, et al. The effects of HIF-1alpha on gene expression profiles of NCI-H446 human small cell lung cancer cells. J Exp Clin Cancer Res 2009;28:150. [Crossref] [PubMed]
- Eliasz S, Liang S, Chen Y, et al. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene 2010;29:2488-98. [Crossref] [PubMed]
- Tung KH, Lin CW, Kuo CC, et al. CHC promotes tumor growth and angiogenesis through regulation of HIF-1alpha and VEGF signaling. Cancer Lett 2013;331:58-67. [Crossref] [PubMed]
- Gharib TG, Chen G, Huang CC, et al. Genomic and proteomic analyses of vascular endothelial growth factor and insulin-like growth factor-binding protein 3 in lung adenocarcinomas. Clin Lung Cancer 2004;5:307-12. [Crossref] [PubMed]
- Brader S, Eccles SA. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori 2004;90:2-8. [Crossref] [PubMed]
- Schlie K, Spowart JE, Hughson LR, et al. When cells suffocate: autophagy in cancer and immune cells under low oxygen. Int J Cell Biol 2011;2011:470597. [Crossref] [PubMed]
- Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 2005;7:1-6. [Crossref] [PubMed]
- Popper HH. Lung adenocarcinomas: comparison between mice and men. Methods Mol Biol 2015;1267:19-43. [Crossref] [PubMed]
- Liu YL, Yu JM, Song XR, et al. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol Ther 2006;5:1320-6. [Crossref] [PubMed]
- Paliwal S, Kovi RC, Nath B, et al. The alternative reading frame tumor suppressor antagonizes hypoxia-induced cancer cell migration via interaction with the COOH-terminal binding protein corepressor. Cancer Res 2007;67:9322-9. [Crossref] [PubMed]
- Monnier Y, Farmer P, Bieler G, et al. CYR61 and alphaVbeta5 integrin cooperate to promote invasion and metastasis of tumors growing in preirradiated stroma. Cancer Res 2008;68:7323-31. [Crossref] [PubMed]
- Wei L, Song XR, Sun JJ, et al. Lysyl oxidase may play a critical role in hypoxia-induced NSCLC cells invasion and migration. Cancer Biother Radiopharm 2012;27:672-7. [Crossref] [PubMed]
- Kim B, Sohn EJ, Jung JH, et al. Inhibition of ZNF746 suppresses invasion and epithelial to mesenchymal transition in H460 non-small cell lung cancer cells. Oncol Rep 2014;31:73-8. [Crossref] [PubMed]
- Nakamoto T, Suzuki T, Huang J, et al. Analysis of gene expression profile in p130(Cas)-deficient fibroblasts. Biochem Biophys Res Commun 2002;294:635-41. [Crossref] [PubMed]
- Kawashiri S, Tanaka A, Noguchi N, et al. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck 2009;31:1346-53. [Crossref] [PubMed]
- Noguchi M, Shimosato Y. The development and progression of adenocarcinoma of the lung. Cancer Treat Res 1995;72:131-42. [Crossref] [PubMed]
- Kargl J, Busch SE, Yang GH, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 2017;8:14381. [Crossref] [PubMed]
- Gregory AD, Kliment CR, Metz HE, et al. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol 2015;98:143-52. [Crossref] [PubMed]
- Krohn A, Ahrens T, Yalcin A, et al. Tumor cell heterogeneity in small cell lung cancer (SCLC): phenotypical and functional differences associated with epithelial-mesenchymal transition (EMT) and DNA methylation changes. PLoS One 2014;9:e100249. [Crossref] [PubMed]
- Xu MH, Gao X, Luo D, et al. EMT and acquisition of stem cell-like properties are involved in spontaneous formation of tumorigenic hybrids between lung cancer and bone marrow-derived mesenchymal stem cells. PLoS One 2014;9:e87893. [Crossref] [PubMed]
- Nakamura N, Iijima T, Mase K, et al. Phenotypic differences of proliferating fibroblasts in the stroma of lung adenocarcinoma and normal bronchus tissue. Cancer Sci 2004;95:226-32. [Crossref] [PubMed]
- Chen H, Yang WW, Wen QT, et al. TGF-beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM Exp Mol Pathol 2009;87:189-94. [corrected]. [Crossref] [PubMed]
- Santos AM, Jung J, Aziz N, et al. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 2009;119:3613-25. [Crossref] [PubMed]
- García-de-Alba C, Becerril C, Ruiz V, et al. Expression of matrix metalloproteases by fibrocytes: possible role in migration and homing. Am J Respir Crit Care Med 2010;182:1144-52. [Crossref] [PubMed]
- Koukourakis MI, Giatromanolaki A, Brekken RA, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res 2003;63:5376-80. [PubMed]
- Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, et al. Colorectal cancer desmoplastic reaction up-regulates collagen synthesis and restricts cancer cell invasion. Cell Tissue Res 2011;346:223-36. [Crossref] [PubMed]
- Oh JJ, Taschereau EO, Koegel AK, et al. RBM5/H37 tumor suppressor, located at the lung cancer hot spot 3p21.3, alters expression of genes involved in metastasis. Lung Cancer 2010;70:253-62. [Crossref] [PubMed]
- Soltermann A, Tischler V, Arbogast S, et al. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res 2008;14:7430-7. [Crossref] [PubMed]
- Khan MA, Assiri AM, Broering DC. Complement and macrophage crosstalk during process of angiogenesis in tumor progression. J Biomed Sci 2015;22:58. [Crossref] [PubMed]
- Makarenkova VP, Shurin GV, Tourkova IL, et al. Lung cancer-derived bombesin-like peptides down-regulate the generation and function of human dendritic cells. J Neuroimmunol 2003;145:55-67. [Crossref] [PubMed]
- Liu Q, Zhang C, Sun A, et al. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol 2009;182:6207-16. [Crossref] [PubMed]
- Xu L, Xu W, Jiang Z, et al. Depletion of CD4(+)CD25(high) regulatory T cells from tumor infiltrating lymphocytes predominantly induces Th1 type immune response in vivo which inhibits tumor growth in adoptive immunotherapy. Cancer Biol Ther 2009;8:66-72. [Crossref] [PubMed]
- Li L, Chao QG, Ping LZ, et al. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm 2009;24:357-67. [Crossref] [PubMed]
- Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011;6:824-33. [Crossref] [PubMed]
- Feng PH, Lee KY, Chang YL, et al. CD14(+)S100A9(+) monocytic myeloid-derived suppressor cells and their clinical relevance in non-small cell lung cancer. Am J Respir Crit Care Med 2012;186:1025-36. [Crossref] [PubMed]
- Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2012;2:722-35. [Crossref] [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Pelosi G, Fraggetta F, Nappi O, et al. Pleomorphic carcinomas of the lung show a selective distribution of gene products involved in cell differentiation, cell cycle control, tumor growth, and tumor cell motility: a clinicopathologic and immunohistochemical study of 31 cases. Am J Surg Pathol 2003;27:1203-15. [Crossref] [PubMed]
- Blaukovitsch M, Halbwedl I, Kothmaier H, et al. Sarcomatoid carcinomas of the lung--are these histogenetically heterogeneous tumors? Virchows Arch 2006;449:455-61. [Crossref] [PubMed]
- Lee HW, Park YM, Lee SJ, et al. Alpha-smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma. Clin Cancer Res 2013;19:5879-89. [Crossref] [PubMed]
- Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011;12:413-26. [Crossref] [PubMed]
- Li CM, Chen G, Dayton TL, et al. Differential Tks5 isoform expression contributes to metastatic invasion of lung adenocarcinoma. Genes Dev 2013;27:1557-67. [Crossref] [PubMed]
- Lin HC, Zhang FL, Geng Q, et al. Quantitative proteomic analysis identifies CPNE3 as a novel metastasis-promoting gene in NSCLC. J Proteome Res 2013;12:3423-33. [Crossref] [PubMed]
- Narita K, Matsuda Y, Seike M, et al. Nestin regulates proliferation, migration, invasion and stemness of lung adenocarcinoma. Int J Oncol 2014;44:1118-30. [Crossref] [PubMed]
- Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927-39. [Crossref] [PubMed]
- Pallier K, Cessot A, Cote JF, et al. TWIST1 a New Determinant of epithelial to mesenchymal transition in EGFR mutated lung adenocarcinoma. PLoS One 2012;7:e29954. [Crossref] [PubMed]
- Pirozzi G, Tirino V, Camerlingo R, et al. Epithelial to mesenchymal transition by TGFbeta-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One 2011;6:e21548. [Crossref] [PubMed]
- Wang C, Su Y, Zhang L, et al. The function of SARI in modulating epithelial-mesenchymal transition and lung adenocarcinoma metastasis. PLoS One 2012;7:e38046. [Crossref] [PubMed]
- Luo J, Zuo J, Wu J, et al. In vivo RNAi screen identifies candidate signaling genes required for collective cell migration in Drosophila ovary. Sci China Life Sci 2015;58:379-89. [Crossref] [PubMed]
- Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer 2019;19:553-67. [Crossref] [PubMed]
- Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013;24:130-7. [Crossref] [PubMed]
- Wu JS, Sheng SR, Liang XH, et al. The role of tumor microenvironment in collective tumor cell invasion. Future Oncol 2017;13:991-1002. [Crossref] [PubMed]
- Richter U, Schroder C, Wicklein D, et al. Adhesion of small cell lung cancer cells to E- and P-selectin under physiological flow conditions: implications for metastasis formation. Histochem Cell Biol 2011;135:499-512. [Crossref] [PubMed]
- Follain G, Herrmann D, Harlepp S, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer 2020;20:107-24. [Crossref] [PubMed]
- Jolly MK, Boareto M, Huang B, et al. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol 2015;5:155. [Crossref] [PubMed]
- George JT, Jolly MK, Xu S, et al. Survival outcomes in cancer patients predicted by a partial EMT gene expression scoring metric. Cancer Res 2017;77:6415-28. [Crossref] [PubMed]
- Lecharpentier A, Vielh P, Perez-Moreno P, et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 2011;105:1338-41. [Crossref] [PubMed]
- Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A 2010;107:21677-82. [Crossref] [PubMed]
- Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019;566:553-7. [Crossref] [PubMed]
- Orellana R, Kato S, Erices R, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer 2015;15:290. [Crossref] [PubMed]
- Leach J, Morton JP, Sansom OJ. Neutrophils: homing in on the myeloid mechanisms of metastasis. Mol Immunol 2019;110:69-76. [Crossref] [PubMed]
- Chennakrishnaiah S, Meehan B, D'Asti E, et al. Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles. J Thromb Haemost 2018;16:1800-13. [Crossref] [PubMed]
- Gil-Bernabé AM, Ferjančič Š, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012;119:3164-75. [Crossref] [PubMed]
- Wahrenbrock M, Borsig L, Le D, et al. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003;112:853-62. [Crossref] [PubMed]
- Ilkovitch D, Carrio R, Lopez DM. uPA and uPA-receptor are involved in cancer-associated myeloid-derived suppressor cell accumulation. Anticancer Res 2012;32:4263-70. [PubMed]
- Takanami I, Takeuchi K, Karuke M. Expression of ETS-1 is correlated with urokinase-type plasminogen activator and poor prognosis in pulmonary adenocarcinoma. Tumour Biol 2001;22:205-10. [Crossref] [PubMed]
- Gabor S, Renner H, Popper H, et al. Invasion of blood vessels as significant prognostic factor in radically resected T1-3N0M0 non-small-cell lung cancer. Eur J Cardiothorac Surg 2004;25:439-42. [Crossref] [PubMed]
- Lin MI, Yu J, Murata T, et al. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res 2007;67:2849-56. [Crossref] [PubMed]
- Hiratsuka S, Goel S, Kamoun WS, et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A 2011;108:3725-30. [PubMed]
- Hiratsuka S, Ishibashi S, Tomita T, et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun 2013;4:1853. [PubMed]
- Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem Soc Trans 2004;32:397-402. [Crossref] [PubMed]
- Ferjančič Š, Gil-Bernabé AM, Hill SA, et al. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood 2013;121:3289-97. [Crossref] [PubMed]
- Holopainen T, Saharinen P, D'Amico G, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst 2012;104:461-75. [Crossref] [PubMed]
- Heidemann F, Schildt A, Schmid K, et al. Selectins mediate small cell lung cancer systemic metastasis. PLoS One 2014;9:e92327. [Crossref] [PubMed]
- Wakisaka N, Hasegawa Y, Yoshimoto S, et al. Primary tumor-secreted lymphangiogenic factors induce pre-metastatic lymphvascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS One 2015;10:e0144056. [Crossref] [PubMed]
- Farnsworth RH, Karnezis T, Shayan R, et al. A role for bone morphogenetic protein-4 in lymph node vascular remodeling and primary tumor growth. Cancer Res 2011;71:6547-57. [Crossref] [PubMed]
- Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019;19:307-25. [Crossref] [PubMed]
- Chen FA, Alosco T, Croy BA, et al. Clones of tumor cells derived from a single primary human lung tumor reveal different patterns of beta 1 integrin expression. Cell Adhes Commun 1994;2:345-57. [Crossref] [PubMed]
- Luzzi KJ, MacDonald IC, Schmidt EE, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 1998;153:865-73. [Crossref] [PubMed]
- Balakrishnan A, Koppaka D, Anand A, et al. Circulating tumor cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep 2019;9:7933. [Crossref] [PubMed]
- Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol 2012;7:512-9. [Crossref] [PubMed]
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Takano K, Kinoshita M, Takagaki M, et al. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro Oncol 2016;18:716-24. [Crossref] [PubMed]
- Hui L, Qing YL, Qian CZ, et al. Brain metastasis as the first manifestation of small cell lung cancer in a female adolescent. Lung Cancer 2011;73:243-6. [Crossref] [PubMed]
- Sadanandam A, Varney ML, Kinarsky L, et al. Identification of functional cell adhesion molecules with a potential role in metastasis by a combination of in vivo phage display and in silico analysis. OMICS 2007;11:41-57. [Crossref] [PubMed]
- Liang Z, Zhan W, Zhu A, et al. Development of a unique small molecule modulator of CXCR4. PLoS One 2012;7:e34038. [Crossref] [PubMed]
- Horak CE, Steeg PS. Metastasis gets site specific. Cancer Cell 2005;8:93-5. [Crossref] [PubMed]
- Tang CH, Tan TW, Fu WM, et al. Involvement of matrix metalloproteinase-9 in stromal cell-derived factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis 2008;29:35-43. [Crossref] [PubMed]
- Raimondi L, De Luca A, Amodio N, et al. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 2015;6:13772-89. [Crossref] [PubMed]
- D'Alterio C, Barbieri A, Portella L, et al. Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol Immunother 2012;61:1713-20. [Crossref] [PubMed]
- Liu B, Wu X, Wang C, et al. MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochim Biophys Acta 2012;1822:1692-704. [Crossref] [PubMed]
- Gutschner T, Hammerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 2013;73:1180-9. [Crossref] [PubMed]
- Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003;22:8031-41. [Crossref] [PubMed]
- Morales S, Velasco A, Gasol A, et al. Circulating tumor cells (CTCs) and cytokeratin 19 (CK19) mRNA as prognostic factors in heavily pretreated patients with metastatic breast cancer. Cancer Treat Res Commun 2018;16:13-7. [Crossref] [PubMed]
- Aceto N, Bardia A, Wittner BS, et al. AR expression in breast cancer CTCs associates with bone metastases. Mol Cancer Res 2018;16:720-7. [Crossref] [PubMed]
- Wang Z, Chen JQ, Liu JL, et al. Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med 2016;14:297. [Crossref] [PubMed]
- Steinbichler TB, Dudas J, Riechelmann H, et al. The role of exosomes in cancer metastasis. Semin Cancer Biol 2017;44:170-81. [Crossref] [PubMed]
- Becker A, Thakur BK, Weiss JM, et al. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 2016;30:836-48. [Crossref] [PubMed]
- Zhou L, Lv T, Zhang Q, et al. The biology, function and clinical implications of exosomes in lung cancer. Cancer Lett 2017;407:84-92. [Crossref] [PubMed]
- Rodrigues G, Hoshino A, Kenific CM, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol 2019;21:1403-12. [Crossref] [PubMed]
- Zeng Z, Li Y, Pan Y, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun 2018;9:5395. [Crossref] [PubMed]
- Gangoda L, Liem M, Ang CS, et al. Proteomic profiling of exosomes secreted by breast cancer cells with varying metastatic potential. Proteomics 2017. [Crossref] [PubMed]
- Ringuette Goulet C, Bernard G, Tremblay S, et al. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFbeta signaling. Mol Cancer Res 2018;16:1196-204. [Crossref] [PubMed]
- Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer 2014;84:86-91. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Fang B. RAS signaling and anti-RAS therapy: lessons learned from genetically engineered mouse models, human cancer cells, and patient-related studies. Acta Biochim Biophys Sin (Shanghai) 2016;48:27-38. [PubMed]
- Musteanu M, Blaas L, Zenz R, et al. A mouse model to identify cooperating signaling pathways in cancer. Nat Methods 2012;9:897-900. [Crossref] [PubMed]
- Nolte SM, Venugopal C, McFarlane N, et al. A cancer stem cell model for studying brain metastases from primary lung cancer. J Natl Cancer Inst 2013;105:551-62. [Crossref] [PubMed]
- Rietkötter E, Bleckmann A, Bayerlova M, et al. Anti-CSF-1 treatment is effective to prevent carcinoma invasion induced by monocyte-derived cells but scarcely by microglia. Oncotarget 2015;6:15482-93. [Crossref] [PubMed]
- Kafka A, Tomas D, Beros V, et al. Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down-regulation of E-cadherin. Int J Mol Sci 2014;15:10635-51. [Crossref] [PubMed]
- Bleckmann A, Siam L, Klemm F, et al. Nuclear LEF1/TCF4 correlate with poor prognosis but not with nuclear beta-catenin in cerebral metastasis of lung adenocarcinomas. Clin Exp Metastasis 2013;30:471-82. [Crossref] [PubMed]
- Jeevan DS, Cooper JB, Braun A, et al. Molecular Pathways Mediating Metastases to the Brain via Epithelial-to-Mesenchymal Transition: Genes, Proteins, and Functional Analysis. Anticancer Res 2016;36:523-32. [PubMed]
- Fábián K, Nemeth Z, Furak J, et al. Protein expression differences between lung adenocarcinoma and squamous cell carcinoma with brain metastasis. Anticancer Res 2014;34:5593-7. [PubMed]
- Yoo JY, Yang SH, Lee JE, et al. E-cadherin as a predictive marker of brain metastasis in non-small-cell lung cancer, and its regulation by pioglitazone in a preclinical model. J Neurooncol 2012;109:219-27. [Crossref] [PubMed]
- Wang L, Cossette SM, Rarick KR, et al. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS One 2013;8:e80933. [Crossref] [PubMed]
- Valiente M, Obenauf AC, Jin X, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014;156:1002-16. [Crossref] [PubMed]
- Lin CY, Chen HJ, Huang CC, et al. ADAM9 promotes lung cancer metastases to brain by a plasminogen activator-based pathway. Cancer Res 2014;74:5229-43. [Crossref] [PubMed]
- Sher YP, Wang LJ, Chuang LL, et al. ADAM9 up-regulates N-cadherin via miR-218 suppression in lung adenocarcinoma cells. PLoS One 2014;9:e94065. [Crossref] [PubMed]
- Preusser M, Berghoff AS, Berger W, et al. High rate of FGFR1 amplifications in brain metastases of squamous and non-squamous lung cancer. Lung Cancer 2014;83:83-9. [Crossref] [PubMed]
- Breindel JL, Haskins JW, Cowell EP, et al. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res 2013;73:5053-65. [Crossref] [PubMed]
- Wang L, Wang Z, Liu X, et al. High-level C-X-C chemokine receptor type 4 expression correlates with brain-specific metastasis following complete resection of non-small cell lung cancer. Oncol Lett 2014;7:1871-6. [Crossref] [PubMed]
- Salmaggi A, Maderna E, Calatozzolo C, et al. CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer Biol Ther 2009;8:1608-14. [Crossref] [PubMed]
- Li F, Sun L, Zhang S. Acquirement of DNA copy number variations in nonsmall cell lung cancer metastasis to the brain. Oncol Rep 2015;34:1701-7. [Crossref] [PubMed]
- Mock K, Preca BT, Brummer T, et al. The EMT-activator ZEB1 induces bone metastasis associated genes including BMP-inhibitors. Oncotarget 2015;6:14399-412. [Crossref] [PubMed]
- Vassella E, Langsch S, Dettmer MS, et al. Molecular profiling of lung adenosquamous carcinoma: hybrid or genuine type? Oncotarget 2015;6:23905-16. [Crossref] [PubMed]
- Biswas D, Birkbak NJ, Rosenthal R, et al. A clonal expression biomarker associates with lung cancer mortality. Nat Med 2019;25:1540-8. [Crossref] [PubMed]
- Shen L, Chen L, Wang Y, et al. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol 2015;121:101-8. [Crossref] [PubMed]
- Zhao N, Wilkerson MD, Shah U, et al. Alterations of LKB1 and KRAS and risk of brain metastasis: comprehensive characterization by mutation analysis, copy number, and gene expression in non-small-cell lung carcinoma. Lung Cancer 2014;86:255-61. [Crossref] [PubMed]
- Pécuchet N, Laurent-Puig P, Mansuet-Lupo A, et al. Different prognostic impact of STK11 mutations in non-squamous non-small-cell lung cancer. Oncotarget 2017;8:23831-40. [Crossref] [PubMed]
- Hwang SJ, Lee HW, Kim HR, et al. Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget 2015;6:20434-48. [Crossref] [PubMed]
- Gao Y, Li G, Sun L, et al. ACTN4 and the pathways associated with cell motility and adhesion contribute to the process of lung cancer metastasis to the brain. BMC Cancer 2015;15:277. [Crossref] [PubMed]
- Risolino M, Mandia N, Iavarone F, et al. Transcription factor PREP1 induces EMT and metastasis by controlling the TGF-beta-SMAD3 pathway in non-small cell lung adenocarcinoma. Proc Natl Acad Sci U S A 2014;111:E3775-84. [Crossref] [PubMed]
- Han L, Liang XH, Chen LX, et al. SIRT1 is highly expressed in brain metastasis tissues of non-small cell lung cancer (NSCLC) and in positive regulation of NSCLC cell migration. Int J Clin Exp Pathol 2013;6:2357-65. [PubMed]
- Paik PK, Shen R, Won H, et al. Next-generation sequencing of stage iv squamous cell lung cancers reveals an association of PI3K aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discov 2015;5:610-21. [Crossref] [PubMed]
- Elzarrad MK, Haroon A, Willecke K, et al. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med 2008;6:20. [Crossref] [PubMed]
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 2004;5:365-74. [Crossref] [PubMed]
- Catena R, Luis-Ravelo D, Anton I, et al. PDGFR signaling blockade in marrow stroma impairs lung cancer bone metastasis. Cancer Res 2011;71:164-74. [Crossref] [PubMed]
- Valencia K, Ormazabal C, Zandueta C, et al. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res 2012;18:969-80. [Crossref] [PubMed]
- Vicent S, Luis-Ravelo D, Anton I, et al. A novel lung cancer signature mediates metastatic bone colonization by a dual mechanism. Cancer Res 2008;68:2275-85. [Crossref] [PubMed]
- Xie L, Yang Z, Li G, et al. Genome-wide identification of bone metastasis-related microRNAs in lung adenocarcinoma by high-throughput sequencing. PLoS One 2013;8:e61212. [Crossref] [PubMed]
- Luis-Ravelo D, Antón I, Zandueta C, et al. RHOB influences lung adenocarcinoma metastasis and resistance in a host-sensitive manner. Mol Oncol 2014;8:196-206. [Crossref] [PubMed]
- Zhang W, Yu C, Huang B, et al. Correlation between bone metastasis and thrombocytosis in pulmonary adenocarcinoma patients. Oncol Lett 2015;9:762-8. [Crossref] [PubMed]
- Nakamura ES, Koizumi K, Kobayashi M, et al. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis 2006;23:9-18. [Crossref] [PubMed]
- Feeley BT, Liu NQ, Conduah AH, et al. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and Rank:Fc administration. J Bone Miner Res 2006;21:1571-80. [Crossref] [PubMed]
- Dougall WC, Holen I, Gonzalez Suarez E. Targeting RANKL in metastasis. Bonekey Rep 2014;3:519. [Crossref] [PubMed]
- Peng X, Guo W, Ren T, et al. Differential expression of the RANKL/RANK/OPG system is associated with bone metastasis in human non-small cell lung cancer. PLoS ONE 2013;8:e58361. [Crossref] [PubMed]
- Miller RE, Jones JC, Tometsko M, et al. RANKL inhibition blocks osteolytic lesions and reduces skeletal tumor burden in models of non-small-cell lung cancer bone metastases. J Thorac Oncol 2014;9:345-54. [Crossref] [PubMed]
- Kuo PL, Liao SH, Hung JY, et al. MicroRNA-33a functions as a bone metastasis suppressor in lung cancer by targeting parathyroid hormone related protein. Biochim Biophys Acta 2013;1830:3756-66. [Crossref] [PubMed]
- Shishido SN, Carlsson A, Nieva J, et al. Circulating tumor cells as a response monitor in stage IV non-small cell lung cancer. J Transl Med 2019;17:294. [Crossref] [PubMed]
- Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res 2018;78:3407-12. [Crossref] [PubMed]
- Sheng M, Dong Z, Xie Y. Identification of tumor-educated platelet biomarkers of non-small-cell lung cancer. Onco Targets Ther 2018;11:8143-51. [Crossref] [PubMed]