
Radiotherapy for non-small cell lung cancer in the immunotherapy era: the opportunity and challenge—a narrative review
Introduction
Radiotherapy is traditionally regarded as a local therapy for cancer that induces deoxyribonucleic acid (DNA) damage in irradiated sites, and it has been a pillar of non-small cell lung cancer (NSCLC) management for many years. Substantial technological improvements have over time led to changes in radiation delivery, including three-dimensional conformal radiotherapy (3D-CRT), intensity modulated radiation therapy (IMRT), stereotactic body radiation therapy (SBRT), and image-guided radiotherapy. However, a combination of radiotherapy and chemotherapy became the standard therapy for patients with stage III NSCLC in the 1990s and has remained so since, resulting in improved local tumor control and survival benefit (1). Interestingly, regression also occurs in tumors situated outside the radiation field in patients with multiple lesions. This is known as the abscopal effect, which was first described in 1953 (2). This phenomenon has been postulated in recent years to be immune mediated (3).
Immune checkpoint inhibitors (ICIs), which target programmed death protein-1 (PD-1), programmed death ligand-1 (PD-L1), or cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), have improved the treatment of patients with NSCLC, although the response rate is limited to 19–47% of patients when the regime is singly administrated (4). Pembrolizumab has been approved as first-line treatment for advanced NSCLC with PD-L1 expression ≥50% on tumor cells. And a combination of pembrolizumab and chemotherapy is also first-line treatment option (5-8). Asides from the ICIs, there are also many other immunotherapies for NSCLC, such as chimeric antigen receptor (CAR) T-cell therapy, exogenous cytokines, vaccines, and antibody-based reagents. Numerous preclinical and clinical trials are therefore exploring radiotherapy and ICIs as a combined therapy to improve patient response rates. An increase in the incidence of abscopal effects has been observed in these combination therapy trials when ICIs are given sequentially or concurrently with radiotherapy (9). In addition, the PACIFIC trial showed that combining chemoradiotherapy with durvalumab could improve clinical outcomes in patients with unresectable stage III NSCLC (10). The latest idea is that radiotherapy should be administrated in doses and fractionations suitable to elicit tumor-targeting immune responses (11). In this review, we summarize recently reported clinical trials that evaluated ICI therapy for patients across different NSCLC disease stages and review the rationale for combining radiotherapy with ICIs. We also provide an overview of data that explores the timing of radiotherapy, optimal doses and fractionations, radiotherapy targets and target volume, acquired resistance, patient selection, and the toxicity of combination treatment. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-827).
Immunotherapy in NSCLC
Checkpoint inhibitors in a metastatic setting
The development of ICIs has fundamentally changed the treatment of patients with metastatic NSCLC. Pembrolizumab, nivolumab, and atezolizumab were initially approved as second-line treatments for patients with metastatic NSCLC after initial treatment with platinum-based chemotherapy (12-15). Researchers then evaluated these regimes in a first-line setting. In the KEYNOTE-024 trial, pembrolizumab was evaluated as first-line treatment for metastatic NSCLC where PD-L1 was expressed on at least 50% of tumor cells (TCs) (7,8) The KEYNOTE 042 trial, which evaluated patients with a PD-L1 tumor proportion score (TPS) ≥1%, confirmed the efficacy of pembrolizumab as a first-line treatment for metastatic NSCLC (16). Similarly, the IMpower 110 trial evaluated atezolizumab as a first-line therapy for advanced NSCLC with PD-L1 expression ≥1% on TCs or immune cells (IC). The interim analysis reported that first-line treatment with atezolizumab improved overall survival (OS) in TC3 or IC3 (PD-L1 expression ≥50% on TC and ≥10% or greater in IC) patients (17). However, first-line treatment of nivolumab failed to improve clinical outcomes in patients with advanced NSCLC (18).
The KEYNOTE-189 study demonstrated that the combination of pembrolizumab and chemotherapy (pemetrexed plus cisplatin or carboplatin) improved progression-free survival (PFS) (8.8 vs. 4.9 months) and 12-month OS (69.2% vs. 49.4%) in advanced non-squamous NSCLC; patients benefited from the treatment regardless of PD-L1 TPS (6). Similarly, the KEYNOTE-407 trial showed that the combination of pembrolizumab and chemotherapy (paclitaxel/nab-paclitaxel plus carboplatin) improved PFS and OS in patients with squamous NSCLC (5). In addition, the IMpower 150 trial showed that the combination of atezolizumab, paclitaxel, and carboplatin with bevacizumab improved PFS and OS compared to the same regimen without atezolizumab in patients with advanced non-squamous NSCLC (19). The IMpower 130 trial showed similar results without the administration of bevacizumab (20). Based on the above large randomized studies, patients without molecular alterations that respond to drug therapy and with PD-L1 TPS ≥50% are treated with either pembrolizumab alone or a combination of pembrolizumab and chemotherapy, and a combination of pembrolizumab and chemotherapy is preferred for patients with PD-L1 TPS <50%. For patients with PD-L1 TPS <50% in non-squamous NSCLC, atezolizumab plus chemotherapy and bevacizumab is another first-line treatment option.
Checkpoint inhibitors for locally advanced and resectable NSCLC
Given the high risk of distant metastasis after definitive radiotherapy for locally advanced NSCLC, many ongoing studies are directed to reducing distant metastasis and improving OS. The PACIFIC trial was a randomized phase III trial comparing durvalumab as consolidation therapy with a placebo in locally advanced unresectable NSCLC where disease did not progress after definitive platinum-based chemoradiotherapy (21). Patients were randomly assigned to receive either durvalumab or a placebo within 42 days after chemoradiotherapy. The median PFS was significantly longer in the durvalumab group compared with the placebo group (17.2 vs. 5.6 months; HR =0.51; 95% CI: 0.41–0.63; P<0.001). An update was given at the 2019 American Society of Clinical Oncology (ASCO) Annual Conference, revealing that the 36-month OS rate was 57% in the durvalumab group and 43.5% n the placebo group (22). LUN 14-179 was a similar phase II trial evaluating the safety and efficacy of pembrolizumab as consolidation therapy in stage III NSCLC patients who had completed chemoradiotherapy (23). The median PFS was 15.4 months and the estimated two-year OS was 68.7%. RTOG 3505 (NCT02768558) is a comparable trial assessing nivolumab as consolidation therapy for patients with unresectable stage III NSCLC. This trial is ongoing and no results have yet been reported (24).
Forde et al. published a prospective phase II trial evaluating the safety and feasibility of nivolumab induction in resectable NSCLC (25). The initial data showed that 20 of 21 tumors were margin-negative resected with a major pathological response (MPR) rate of 45% (9/20) and a pathologic downstaging rate of 40% (8/20). To improve the MPR rate of the neoadjuvant therapy, Provencio et al. conducted a phase II multicenter trial (NADIM) to explore the efficacy of neoadjuvant nivolumab combined with carboplatin and paclitaxel before surgery in stage IIIA resectable NSCLC (26). The combined induction therapy resulted in 34 patients (83%) with an MPR and 24 patients (71%) with a complete pathologic response (CPR). There are also many ongoing trials evaluating the efficacy and safety of combining radiotherapy with immunotherapy as neoadjuvant therapy for resectable NSCLC before surgery, but no results have yet been reported.
Several retrospective studies have demonstrated that postoperative radiotherapy (PORT) with modern techniques (3D-CRT/IMRT) in locally advanced (N2) NSCLC contributed to survival benefit (27-29). However, owing to limited improvement in OS, use of PORT is controversial (30,31) and it remains to be seen whether PORT for locally advanced (N2) NSCLC is essential when neoadjuvant or adjuvant immunotherapy is administrated.
Four phase III ongoing trials are assessing the efficacy and safety of immunotherapy as adjuvant treatment in patients with completely resected stage IB-IIIA NSCLC (NCT02504372, NCT02273375, NCT02595944, and NCT02486718). For patients with early-stage NSCLC unfit for surgical resection, or patients who are unwilling to receive surgery, SBRT is now the standard treatment, although distant relapse occurs in 20% of patients (32). Therefore, the administration of ICIs following SBRT may improve long-term outcome. PACIFIC4 is an ongoing phase III randomized trial evaluating the efficacy and safety of durvalumab following SoC (standard of care) SBRT in patients with unresectable early-stage (T1 to T3N0M0) NSCLC.
Opportunity for combining radiotherapy with immunotherapy
Radiation is a double-edged sword that can both stimulate and suppress the immune system. It induces an immunosuppressive environment via the recruitment of tumor associated macrophages (TAMs), immunosuppressive regulatory T (Treg) cells and myeloid derived suppressor cells (MDSCs) (33,34). Radiation can also increase the secretion of transforming growth factor-β (TGF-β) and hypoxia-inducible factor 1-α (HIF-1α), which inhibits dendritic cell (DC) maturation and induces radioresistance in endothelial cells (35,36).
Importantly, radiation not only breaks the DNA strands of TCs, causing cell death, but it also stimulate the immune system through numerous pathways. Briefly, radiation can expose previously hiddentumor-associated antigens (TAAs) and induce the release of immunostimulatory molecules, such as damage-associated molecular patterns (DAMPs), adenosine triphosphate (ATP), calreticulin, and high mobility group protein 1 (HMGB1), prompting the maturation of DCs and the activation of antitumor T cells (4,37,38). Cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS) detects DNA damage, triggering the synthesis of cyclic GMP-AMP (cGAMP), which modulates the stimulator of interferon genes (STING) protein (39). Through a series of phosphorylation reactions, STING induces the expression of cytokines, such as type 1 interferons (IFNs), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (40). Radiation can also remodel the vasculature of the tumor bed and affect the recruitment of lymphocytes to the tumor (41). Furthermore, radiation can also facilitate the destruction of lesions outside the irradiated field, known as the abscopal effect (42).
Numerous preclinical studies have suggested that radiotherapy and ICIs in combination can systemically eradicate disease in mouse models (43,44). Deng et al. showed that irradiation increased PD-L1 expression in the tumor microenvironment and that the combination of radiation and anti-PD-L1 not only effectively controlled local tumor growth, but also delayed tumor growth in distant sites by activating cytotoxic T cells and reducing the accumulation of MDSCs in mice (45). Radiation in combination with PD-1 blockade in mouse models of NSCLC resulted in the synergistically enhanced antitumor activity through the infiltration of CD8+ T cells (46,47). A retrospective study suggested that tumoral PD-L1 expression increased after chemoradiotherapy in NSCLC, providing pathologic rationale for the administration of ICIs following chemoradiotherapy (48). Therefore, the underlying mechanism for combination therapy is that radiation can active the immune system against TCs. ICIs can then reverse the immunosuppressive effects of the tumor microenvironment by blocking checkpoints.
As discussed above, preclinical studies suggest that radiotherapy an ideal partner for ICIs. There are also many clinical trials that are assessing the efficacy of this combined therapy. A secondary analysis of the phase I KEYNOTE-001 trial suggested that PFS (HR =0.56; 95% CI: 0.34–0.91; P=0.019) and OS (HR =0.58; 95% CI: 0.36–0.94; P=0.026) were significantly longer in patients who had received any prior radiation treatment compared with patients who had not (49). The median PFS was 6.3 months in the prior radiation group compared with 2 months in the no prior radiation group; and the median OS was 10.7 months in the prior radiation group compared with 5.3 months in the no prior radiation group. Of the patients who had received any prior radiotherapy (43% of patients), 39% received extracranial radiotherapy and 25% received thoracic radiotherapy. Further analysis showed that patients who had received prior extracranial radiotherapy had significantly longer PFS (6.3 vs. 2.0 months; P=0.0084) and OS (11.6 vs. 5.3 months; P=0.034) than patients who did not receive extracranial radiotherapy.
Most recently, Theelen et al. published a phase II study in which SBRT was administered to a single tumor lesion in patients with advanced NSCLC, followed by pembrolizumab. This investigated whether SBRT administered before pembrolizumab could enhance tumor response (50). Of the 92 patients enrolled, 76 were randomly assigned to receive only pembrolizumab (the control arm), or to receive pembrolizumab after radiotherapy (the experimental arm). In the experimental arm, pembrolizumab was administrated within seven days of completing SBRT (24 Gy in three fractions). The objective response rate (ORR) at 12 weeks doubled in these patients (18% vs. 36%; P=0.07). The median PFS (1.9 vs. 6.6 months; P=0.19) and OS (7.6 vs. 15.9 months; P=0.16) also improved in experimental arm patients compared with control arm patients. Subgroup analysis suggested that patients with PD-L1-negative tumors had significantly longer PFS and OS, indicating that radiation may alter the tumor microenvironment of PD-L1-negative tumors, facilitating the effects of pembrolizumab.
A systemic review evaluated the efficacy of combining SBRT with ICIs (51). The ICI-SBRT combination had more improved distant abscopal response rates than ICI alone, suggesting that non-responders to ICI therapy were turned into responders by radiotherapy. However, a pooled analysis did not demonstrate a general increase in OS and PFS. Further studies should therefore focus on improving patient selection for this combination therapy. Taken together, these studies suggest that combining radiotherapy with immunotherapy can enhance immune response, though research remains to be done with respect to optimal dose and fractionations, target regions, the sequence of radiotherapy and immunotherapy, and combination therapy toxicity. Many more ongoing clinical trials are investigating the efficacy and safety of different combinations of radiotherapy and ICIs in patients with NSCLC (Tables 1,2).
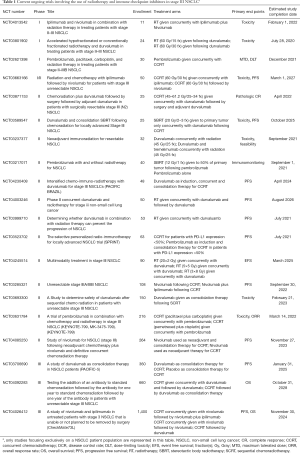
Full table
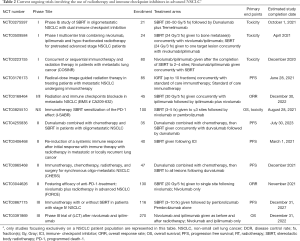
Full table
Challenges for combining radiotherapy with immunotherapy
Timing of radiation therapy
At present, the optimal timing and sequencing of radiotherapy and immunotherapy is unknown. A review has shown that the optimal sequencing of combined immunotherapy and radiotherapy depends on the mechanisms of activation that the immunotherapy facilitates (52). The PACIFIC trial demonstrated that adjuvant administration of durvalumab after chemoradiotherapy improved PFS and OS (10). The clinical outcomes of different subgroups, based on time since completing radiation therapy (RT), to durvalumab were reported at the 2018 European Society for Medical Oncology (ESMO) Annual Meeting (53). PFS was longer in durvalumab group compared to the placebo group regardless of time since completing radiation therapy [<14 days: median not reached (NR) vs. 4.8 months, HR =0.39; ≥14 days: median 14.0 vs 5.6 months, HR =0.63]. However, there was a trend of PFS being longer in the 14 days group. Multivariate analysis showed that the administration of durvalumab within 14 days correlated with better OS. Similarly, pembrolizumab and nivolumab have been administrated as adjuvant therapy after chemoradiotherapy in clinical trials assessing the safety and efficacy of PD-1-targeting ICIs as consolidation therapy (23,24).
However, another retrospective study reported a different optimal time for commencement of immunotherapy after radiotherapy (54). This study recruited patients with metastatic NSCLC treated with SBRT from between 2004 and 2015 from the National Cancer Database (NCDB). A total of 13,862 patients met the study eligibility criteria, and 371 of these patients were treated with immunotherapy after SBRT. Patients treated with immunotherapy at least 21 days after commencing SBRT had longer OS compared to those who received immunotherapy within 21 days after commencing SBRT. The median OS was 19 months and 15 months, respectively (P=0.0335). However, this is a retrospective study with many confounding factors. For example, the tumor burden and the performance state of patients were imbalanced between the two groups. Therefore, the optimal time to receive immunotherapy after radiotherapy still requires exploration through large randomized clinical trials. However, the combined effect of radiotherapy and immunotherapy is not only associated with resting time between the two, but also with the sequence they are administered.
Sequential administration of radiotherapy followed by immunotherapy having increased patient survival benefit, several studies began to explore whether concurrent administration would yield better results. Preclinical research has shown that concurrent administration of immunotherapy and radiotherapy can improve survival in mice models (55). The phase II DETERRED trial evaluated the feasibility of atezolizumab combined with chemoradiotherapy in locally advanced NSCLC (56). This study was designed in two parts. Part 1 consisted of treatment with chemoradiotherapy, followed by consolidation chemotherapy and atezolizumab three weeks after receiving chemoradiotherapy, and then maintenance atezolizumab for up to one year. Once atezolizumab was deemed safe as consolidation and maintenance therapy, part 2 began to enroll patients. This part of the study introduced atezolizumab at the same time as chemoradiotherapy, followed by the same schedule as part 1. In part 1, the median PFS was 18.6 months and the OS was 22.8 months. In part 2, the median PFS was 13.2 months and OS was not reached. Immune-related adverse events (irAEs) of grade 3 or higher had an incidence rate of 20% to 30%, and pneumonitis of grade 2 or higher had an incidence rate of 10% to 16%. Therefore, it was found to be safe and feasible to combine atezolizumab with chemoradiotherapy, though there is no striking improvement in survival compared with the PACIFIC trial results.
After the success of the PACIFIC trial, researchers began to explore the benefits of durvalumab and radiotherapy administered concurrently. PACIFIC 2 (NCT03519971) is an ongoing phase III randomized multicenter study designed to assess the benefit and safety of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable stage III NSCLC (57). Patients will be randomized to receive either durvalumab and chemoradiotherapy or a placebo and chemoradiotherapy, followed by either durvalumab or a placebo in patients without disease progression. The primary endpoints are PFS and ORR. We eagerly await the results of the trial. Similarly, NICOLAS, a phase II trial, assessed the safety and efficacy of nivolumab administered concurrently with chemoradiotherapy, followed by nivolumab treatment for one year in locally advanced NSCLC (58).
As existing data supports immunotherapy as neoadjuvant therapy before surgery, it is postulated that immunotherapy performed before radiotherapy may also have clinical benefits. To evaluate whether atezolizumab administered before chemoradiotherapy can improve outcomes in unresectable stage III NSCLC, Ross et al. is conducting a single-arm, phase II trial (AFT-16, NCT03102242) in which atezolizumab is administered before and after definitive chemoradiotherapy in patients with locally advanced unresectable NSCLC (59). Patients will receive four cycles of atezolizumab before definitive chemoradiotherapy with restaging after cycles 2 and 4, followed by chemoradiotherapy and two cycles of carboplatin and paclitaxel consolidation in non-progressing patients. Patients will then receive atezolizumab for one year. Disease control rate after neoadjuvant atezolizumab is the primary endpoint. A quantitative system pharmacology model that included tumor size, dynamics, and immune markers demonstrated that anti-PD-L1 administration either prior to or concurrently with radiotherapy may produce better synergistic effects (60). Many trials investigating different treatment combination sequences are ongoing and the optimal combinations that improve clinical outcomes in patients will be established in the future.
Dose and fractionation
With the advancement of technology, hypofractionated radiotherapy has become widely administrated in patients with relatively small target volumes in NSCLC. However, calls to increase radiotherapy dose conflict with the notion that an ideal dose of radiotherapy should induce inflammatory TC apoptosis and activate anticancer responses to produce a long-term response (61,62). Preclinical trials have demonstrated that high-dose radiation may result in increased infiltration of tumor-specific CD8+ T cell, upregulation of Fas or intercellular adhesion molecule (ICAM), and enhanced expression of tumor-associated peptides (63-65). However, a fractionated dose of 24 Gy per three fractions can induce an efficient abscopal effect when treated simultaneously with anti-CTLA-4 antibody, while a single dose of 20 Gy cannot (44). Hypofractionated radiotherapy induces the accumulation of endogenous cytosolic DNA, which in turn brings about the activation of the human STING (hSTING) pathway, catalyzing the recruitment of ICs (66). The exonuclease TREX1, which degrades cytosolic DNA, is induced by radiotherapy doses higher than 12–18 Gy (66). It is accepted at present that a radiation dose of between 8 and 10 Gy per fraction in one to two fractions seems to be the optimal dose which induces an effective antitumor response (62).
Based on the results of preclinical experiments and case reports, a wide range of radiation doses and fractionation schedules are being explored in current clinical trials . A subgroup analysis of the PACIFIC trial found that patients enjoyed significant survival benefit regardless of the total radiation dose prescribed in conventional radiotherapy (53). A phase I–II trial combining pembrolizumab administered concurrently with or without thoracic radiation in metastatic NSCLC was reported at the 2019 ASCO Annual Conference. The radiation doses administered were 50 Gy per four fractions, 70 Gy per 10 fractions, or 45 Gy per 15 fractions. The pembrolizumab plus thoracic radiation group did not show a significantly improve response rate outside the irradiated field (22% vs. 25%; P=1.00). However, an exploratory analysis showed that SBRT improved PFS compared with conventional radiotherapy (21.1 vs. 6.8 months; P=0.03), suggesting SBRT may improve response to immunotherapy (67).
A retrospective study showed that patients with untreated melanoma brain metastases who received fractionation at 3×9 Gy had better intracranial PFS than those who received a single-fraction dose of between 18 and 20 Gy (70% vs. 46% at 6 months; P=0.01), especially when combined with nivolumab (68), as previously reported in preclinical tumor models (44). Another clinical trial (NCT02221739) explored the combination of radiotherapy and ipilimumab in chemotherapy-refractory metastatic patients with NSCLC (69). Either five fractions of 6 Gy or three fractions of 9 Gy were administrated to a single site of metastasis. The results demonstrated that both radiation regimens increased treatment response, and no significant difference in treatment response was found between them.
There are currently no significant clinical trials that demonstrate the optimal dose and fractionation of radiotherapy in combination with immunotherapy. However, we can speculate that radioimmunotherapy might require lower doses of radiation than the maximum tolerated dose, which provides an opportunity to combine a lower dose of radiation with immunotherapy (70).
RT target and target volume
Radiation doses of 60 Gy are conventionally administrated for gross tumor eradication, and prophylactic doses of 45–50 Gy are used for subclinical disease (71,72). As DLN are important sites of activation and accumulation of antitumor T lymphocytes (73), elective nodal irradiation (ENI) may affect the adaptive immune response. Accordingly, by irradiating the DLN, the adaptive immune response was attenuated in a transplantable mouse model treated with stereotactic radiotherapy and ENI, especially when radiotherapy and immunotherapy were combined (74). However, there are no clinical trials currently evaluating DLN irradiation with combined radiotherapy and immunotherapy in locally advanced NSCLC.
Tang et al. suggested that significant predictors of lymphopenia are the volume of lung tissue that receives 5 Gy of radiation and gross tumor volume; lymphopenia can strongly impact clinical outcomes in patients (75). Additionally, peripheral lymphocytes are radio-sensitive cells with a D50 as low as 2 Gy (76). Conventional radiation of 30 fractions can deliver at least 0.5 Gy exposure to 99% of circulating cells (77). Indeed, a systemic review showed that radiation-induced lymphopenia (RIL) was an independent predictor of OS (78). In addition, several studies have demonstrated that the mean radiation dose received by the heart, lungs, and body, and estimated dose of radiation to immune cells (EDRIC), correlated with lymphopenia (75,79-81). Therefore, appropriate volumes of tissue to be irradiated should be defined. We recommend SBRT or proton therapy to reduce mean body dose and minimize RIL.
A preclinical study has demonstrated that radiotherapy can elicit a tumor response when parts of the tumor receive radiation (82). Similarly, a phase I study that evaluated the safety of multisite SBRT followed by pembrolizumab in patients with metastatic solid tumors showed that disease control at three months was not significantly different between patients with partial and complete irradiation (83). Metastases volumes greater than 65 mL were partially treated with SBRT, and the median gross tumor volumes were 116.6 mL for the partially irradiated group and 7.2 mL for entirely irradiated group. However, the results of this trial should be considered with caution, because tumor regions excluded by SBRT planning also received low doses of radiotherapy, which contributed to tumor response in metastatic tumors partially treated with radiotherapy (84). Moreover, only 17 of the 68 patients received partial irradiation, and the follow-up time was too short to obtain an OS (85).
Many clinical trials have explored the efficacy of irradiation of a single lesion combined with ICI since a case report described the abscopal effect in a melanoma patient who was treated with ipilimumab and radiotherapy (42). However, emerging evidence suggests that this strategy does not produce a substantial abscopal effect or any clinical benefits (86).
Considering the distinct TAAs of different metastases, the genomic heterogeneity of tumors, and tumor burden, the irradiation of multiple sites instead of a single site has been suggested to enhance the efficacy of radiotherapy combined with ICI (4). Furthermore, irradiation of specific metastatic lesions should be based on the feasibility and safety of the radiation. For example, SBRT is tolerated by lung parenchyma and liver tissues (4). A phase I trial that evaluated the safety and efficacy of SBRT combined with ipilimumab in lung and liver metastases in NSCLC suggested that liver SBRT resulted in greater T cell activation than lung SBRT, in turn producing more favorable patient outcomes (87).However, the bone marrow is a site of immune privilege, which is a phenomenon where certain sites are less able to develop an antitumor response after RT (88,89). Therefore, the irradiation of bone is not able to enhance the efficacy of ICI.
Acquired resistance
Although ICIs revolutionized the treatment of NSCLC, only 20% of unselected patients with advanced disease respond to PD-1/PD-L1 inhibitors, and 45% of selected patients with PD-L1 expression on at least 50% of TCs are susceptible to PD-1/PD-L1 inhibitors (7,90). When patients have no objective tumor response to their initial therapy with PD-1/PD-L1 inhibitors, this is called primary resistance (91). The duration of response to ICIs ranges from 12 to 25 months in advanced NSCLC, with a small percentage of patients having a durable response lasting more than two years (92). When patients develop progressive disease despite responding to their initial treatment with ICIs, this is called acquired resistance (93). Primary and acquired immunotherapy resistance affects the efficiency of ICIs, which underscores the urgency to develop more effective strategies to overcome such resistance.
Neoantigen loss, the mutation of janus kinase 1 (JAK1)/janus kinase 2 (JAK2), epigenetic stability of exhausted T cells, and the upregulation of inhibitory immune checkpoints can occur in acquired resistance (94-97). A study that characterized the clinical patterns of acquired resistance to PD-1/PD-L1 inhibitors in patients with advanced NSCLC demonstrated that acquired resistance was limited to one or two sites, lymph nodes being the most common site of acquired resistance (92). Local therapy, such as radiotherapy, was given to 15 patients who experienced acquired resistance, and the two-year survival rate of these patients was 92% (95% CI: 0.77–1.00). Eleven patients continued taking ICIs after local therapy. Therefore, it was found that patients who develop oligo-progression after responding to ICIs can receive radiotherapy to prolong ICI effectiveness.
Although the combination of radiotherapy and immunotherapy has improved clinical outcomes, as demonstrated in many clinical trials, most patients eventually relapse and develop acquired resistance (98,99). Chronic IFN-γ activation, the conversion of ATP to adenosine, extra-cellular matrix remodeling and infiltration of Tregs, MDSCs, and macrophages suppress the response to this combination therapy (100). Understanding the mechanism of acquired resistance to combination therapy provides a foundation for developing strategies to overcome this resistance. It is essential to design preclinical and clinical trials that target components in the tumor microenvironment to reduce immunosuppression.
Patient selection
Significant effort is put into selecting suitable patients for combination therapy. However, a reliable predictive marker or model of responses to immunotherapy or combination regimens remains unavailable. Local or distant progression frequently occurs after chemoradiation for inoperable NSCLC. Due to the heterogeneity of these patients, the 5-year survival rate ranges from 6% to 30% (101). The RTOG 0617 randomized trial showed that clinical outcomes did not improve in their dose escalation group, suggesting that local intensification of RT dose may not be a feasible treatment for all patients with inoperable NSCLC (102). Therefore, establishing a predictive model to assess failure patterns in inoperable NSCLC can further guide the individualization of radioimmunotherapy treatment options.
Research has shown that PD-L1 expression, tumor mutational burden, DNA mismatch-repair deficiency, interferon-γ-related gene expression, and tumor-infiltrating lymphocytes are potential biomarkers for ICIs (103,104). In the PACIFIC trial, a post-hoc exploratory analysis showed that durvalumab as consolidation therapy significantly improved PFS and OS compared with a placebo in people with ≥1% PD-L1 expression (HR =0.46; 95% CI: 0.33–0.61) (10). However, in patients with <1% PD-L1 expression, PFS and OS did not significantly improve in the durvalumab group compared with the placebo group. As the subgroup analysis was not representative of the patients in the trial and the numbers of patients with PD-L1 expression <1% was low, the efficacy of durvalumab in patients with PD-L1 expression <1% should be explored in the future.
Radiomics and similar machine-learning approaches based on using imaging data to predict response to immunotherapy also stand out as promising approaches to noninvasively assess tumor-infiltrating CD8+ T cells (105). Radiomics strategies provide longitudinal monitoring of tumor characteristics. When combined with tumor biopsies and genomics, this could improve patient selection. Considering the diversity and complexity of combination therapy, suitable biomarkers should be found at multiple levels, including the genome, transcriptome, proteome, metabolome, and microbiome levels (106). Circulating ICs, circulating cytokines, and circulating antitumor autoantibodies in the peripheral blood which can be frequently sampled could also be used as biomarkers to predict patient response to radiotherapy and radioimmunotherapy (107). In summary, there is not currently any one biomarker that can successfully predict a patient’s response to combination therapy, and all existing potential biomarkers need to be validated in clinical trials.
Toxicity of combination therapy
Immunotherapy given alone can cause adverse effects (AEs) such as rash, diarrhea, fatigue, immune-related pneumonia, and myocarditis (a rare AE), et cetera. A meta-analysis to assess the incidence of irAEs in NSCLC patients receiving PD-1 or PD-L1 inhibitors included 10 trials of PD-1 inhibitors and six trials of PD-L1 inhibitors. This analysis showed that the incidence of irAEs was 22% for all irAE grades, and only 4% for grade 3-4 irAEs (108). It took a median of 10 weeks from the start of treatment for irAEs to occur.
The combination of radiotherapy and immunotherapy not only modulates immune response, but also affect the type and severity of treatment-related AEs (109). Some retrospective research has suggested that radiotherapy and ICIs in combination did not result in increased toxicity (110-112). A retrospective cohort study that examined patients with metastatic lung cancer who were treated with anti-PD-1/PD-L1 therapy suggested that the incidence of grade 2 or more irAEs (13.7% vs. 15.4%; P=0.83), all grades of pneumonitis (8.2% vs. 5.5%; P=0.54), and grade 2 or more pneumonitis (4.1% vs. 3.3%; P>0.99) were not significantly different between the radiotherapy and non-radiotherapy cohorts (110). A limitation in this study is that the median time between radiotherapy and anti-PD-1/PD-L1 therapy was 8.6 months.
Vansteenkiste et al. recently studied whether pneumonitis occurring in stage III NSCLC influenced the efficacy of durvalumab in the PACIFIC trial. Grade 3 or 4 AEs occurred in 30.5% of patients treated with durvalumab and 26.1% of those who received a placebo. Pneumonitis or radiation pneumonitis of any grade occurred in 161 (33.9%) patients in the durvalumab group and 58 (24.8%) patients in the placebo group. Pneumonitis was the most common grade 3 or 4 AE, occuring in 3.6% of patients in the durvalumab group and 3% of patients in placebo group, and pneumonitis caused death in 3.1% (5/161) of the durvalumab group and 8.6% (5/58) of the placebo group. Adjusting for the presence of time-dependent pneumonitis in the Cox proportional-hazards model, it was found that OS, PFS, and time to distant metastasis (TTDM) aligned with the results from the intent to treat (ITT) population. In addition, the presence of time-dependent pneumonitis was not significant (P>0.1) in any model. Therefore, it was not found that the presence of pneumonitis influenced any benefit mediated by durvalumab (113).
The LUN14-197 trial, which administered pembrolizumab as consolidation therapy for chemoradiotherapy, showed that 5.4% of patients experienced grade 3–4 pneumonitis and 5.4% of patients experienced grade 3–4 dyspnea (23). The NICOLAS trial showed that anaemia (47.5%), fatigue (45%), and pneumonitis (42.5%) were the most frequent AEs when nivolumab combined with concurrent chemoraidothrapy (58). Grade 3 or 4 pneumonitis did not occur by three months after the end of RT. Therefore, nivolumab combined with concurrent chemoradiotherapy in stage III NSCLC was found to be safe and tolerable, but larger trials are required in the future to verify these results. When combining pembrolizumab with SBRT, the most common AEs were fatigue (39%), flulike symptoms (32%), and a cough (39%) (50). Pneumonia occurred more frequently in the combination group than in the pembrolizumab alone group [3/37 (8%) vs. 9/35 (26%)]. Grade 3–5 pembrolizumab-related toxic effects occurred in 12 patients (17%) and there were no significant differences between two groups.
Over the past few years, many case reports and single-institution retrospective studies have researched the safety of combination radiotherapy and immunotherapy. In addition, numerous clinical trials investigating the combination treatment have begun, most of which are ongoing. Pending further validation, for now we can conclude that combination radiotherapy and immunotherapy seems safe and acceptable.
Future directions
Based on numerous preclinical and clinical research, combining radiotherapy with ICIs is predicted to be an effective treatment model in the future. At present, a growing body of ongoing clinical trials are investigating a range of treatment options, including diverse immunotherapy regimes, various doses and fractionation schedules, smaller target lesions, and different combination treatment sequences. In the future, these clinical trials are expected to offer conclusive evidence that patients with NSCLC can benefit from combination treatment. In addition, clinical trials are also exploring combination treatment in patients at different stages of NSCLC. First, the excellent tolerability profile of ICIs and SBRT provide an opportunity to develop combined strategies in patients with early stage NSCLC who are unfit or unwilling to undergo surgical resection. Second, the efficacy and safety of immunotherapy administrated as neoadjuvant therapy for resectable NSCLC allows the exploration of combining radiotherapy with immunotherapy as neoadjuvant therapy before surgery. Third, radiotherapy given concurrently with immunotherapy may increase patient survival compared with immunotherapy as consolidation therapy in locally advanced NSCLC. Finally, SBRT administered to multiple sites concurrently with ICIs may enhance antitumour efficacy in patients with metatatic NSCLC.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (2016YFC0905502), the Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1016A), and the Project of Shanghai Science and Technology Commission (18YF1421500).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-827
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-827). The authors report grants from the National Key Research and Development Program of China (2016YFC0905502), grants from the Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1016A), grants from the Project of Shanghai Science and Technology Commission (18YF1421500), during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Ruiz-Bañobre J, Areses-Manrique MC, Mosquera-Martínez J, et al. Evaluation of the lung immune prognostic index in advanced non-small cell lung cancer patients under nivolumab monotherapy. Transl Lung Cancer Res 2019;8:1078-85. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Pacheco JM, Gao D, Camidge DR. Extended follow-up on KEYNOTE-024 suggests significant survival benefit for pembrolizumab in patients with PD-L1 ≥50%, but unanswered questions remain. Ann Transl Med 2019;7:S127. [Crossref] [PubMed]
- Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18:313-22. [Crossref] [PubMed]
- Bang A, Schoenfeld JD, Sun AY. PACIFIC: shifting tides in the treatment of locally advanced non-small cell lung cancer. Transl Lung Cancer Res 2019;8:S139-46. [Crossref] [PubMed]
- Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res 2018;24:259-65. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Weinstock C, Khozin S, Suzman D, et al. U.S. Food and Drug Administration Approval Summary: Atezolizumab for Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4534-9. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Spigel D, De Marinis F, Giaccone G, et al. IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Annals of Oncology 2019;30:v915. [Crossref]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Gray JE, Villegas AE, Daniel DB, et al. Three-year overall survival update from the PACIFIC trial. J Clin Oncol 2019;37:abstr 8526.
- Durm GA, Althouse SK, Sadiq AA, et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. J Clin Oncol 2018;36:abstr 8500.
- Gerber DE, Urbanic JJ, Langer C, et al. Treatment Design and Rationale for a Randomized Trial of Cisplatin and Etoposide Plus Thoracic Radiotherapy Followed by Nivolumab or Placebo for Locally Advanced Non-Small-Cell Lung Cancer (RTOG 3505). Clin Lung Cancer 2017;18:333-9. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemo-immunotherapy for the treatment of stage IIIA resectable non-small-cell lung cancer (NSCLC): A phase II multicenter exploratory study—Final data of patients who underwent surgical assessment. J Clin Oncol 2019;37:abstr 8509.
- Urban D, Bar J, Solomon B, et al. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. J Thorac Oncol 2013;8:940-6. [Crossref] [PubMed]
- Mikell JL, Gillespie TW, Hall WA, et al. Postoperative radiotherapy is associated with better survival in non-small cell lung cancer with involved N2 lymph nodes: results of an analysis of the National Cancer Data Base. J Thorac Oncol 2015;10:462-71. [Crossref] [PubMed]
- Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol 2015;33:870-6. [Crossref] [PubMed]
- Le Pechoux C, Dunant A, Pignon JP, et al. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. J Clin Oncol 2007;25:e10-1. [Crossref] [PubMed]
- Perry M, Kohman L, Bonner J, et al. Updated analysis of a phase III study of surgical resection and chemotherapy (paclitaxel/carboplatin) (CT) with or without adjuvant radiation therapy (RT) for resected stage III non-small cell lung cancer (NSCLC) CALGB 9734. J Clin Oncol 2005;23:7145. [Crossref]
- van den Berg LL, Klinkenberg TJ, Groen HJ, et al. Patterns of Recurrence and Survival after Surgery or Stereotactic Radiotherapy for Early Stage NSCLC. J Thorac Oncol 2015;10:826-31. [Crossref] [PubMed]
- Manukian G, Bar-Ad V, Lu B, et al. Combining Radiation and Immune Checkpoint Blockade in the Treatment of Head and Neck Squamous Cell Carcinoma. Front Oncol 2019;9:122. [Crossref] [PubMed]
- Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017;14:399-416. [Crossref] [PubMed]
- Liu Y, Xia T, Zhang W, et al. Variations of circulating endothelial progenitor cells and transforming growth factor-beta-1 (TGF-beta1) during thoracic radiotherapy are predictive for radiation pneumonitis. Radiat Oncol 2013;8:189. [Crossref] [PubMed]
- Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004;5:429-41. [Crossref] [PubMed]
- Torihata H, Ishikawa F, Okada Y, et al. Irradiation up-regulates CD80 expression through two different mechanisms in spleen B cells, B lymphoma cells, and dendritic cells. Immunology 2004;112:219-27. [Crossref] [PubMed]
- Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012;12:860-75. [Crossref] [PubMed]
- Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 2018;215:1287-99. [Crossref] [PubMed]
- Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 2016;17:1142-9. [Crossref] [PubMed]
- Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002;62:1462-70. [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Demaria S, Pilones KA, Formenti SC, et al. Exploiting the stress response to radiation to sensitize poorly immunogenic tumors to anti-CTLA-4 treatment. Oncoimmunology 2013;2:e23127. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Herter-Sprie GS, Koyama S, Korideck H, et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 2016;1:e87415. [Crossref] [PubMed]
- Gong X, Li X, Jiang T, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1085-97. [Crossref] [PubMed]
- Yoneda K, Kuwata T, Kanayama M, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer 2019;121:490-6. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Chicas-Sett R, Morales-Orue I, Castilla-Martinez J, et al. Stereotactic Ablative Radiotherapy Combined with Immune Checkpoint Inhibitors Reboots the Immune Response Assisted by Immunotherapy in Metastatic Lung Cancer: A Systematic Review. Int J Mol Sci 2019;20:2173. [Crossref] [PubMed]
- Gunderson AJ, Young KH. Exploring optimal sequencing of radiation and immunotherapy combinations. Adv Radiat Oncol 2018;3:494-505. [Crossref] [PubMed]
- Faivre-Finn C, Spigel DR, Senan S, et al. Efficacy and safety evaluation based on time from completion of radiotherapy to randomization with durvalumab or placebo in pts from PACIFIC. Ann Oncol 2018;29:viii488-92.
- Wegner RE, Abel S, Hasan S, et al. Time from stereotactic body radiotherapy to immunotherapy as a predictor for outcome in metastatic non small cell lung cancer. J Clin Oncol 2019;37:abstr 9024.
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Lin SH, Lin Y, Yao L, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol 2020;15:248-57. [Crossref] [PubMed]
- Bradley JD, Nishio M, Okamoto I, et al. PACIFIC-2: Phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J Clin Oncol 2019;37:abstr TPS8573.
- Peters S, Felip E, Dafni U, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer 2019;133:83-7. [Crossref] [PubMed]
- Ross HJ, Kozono DE, Urbanic JJ, et al. Phase II trial of atezolizumab before and after definitive chemoradiation for unresectable stage III NSCLC. J Clin Oncol 2018;36:abstr TPS8585.
- Kosinsky Y, Dovedi SJ, Peskov K, et al. Radiation and PD-(L)1 treatment combinations: immune response and dose optimization via a predictive systems model. J Immunother Cancer 2018;6:17. [Crossref] [PubMed]
- Galluzzi L, Buque A, Kepp O, et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28:690-714. [Crossref] [PubMed]
- Buchwald ZS, Wynne J, Nasti TH, et al. Radiation, Immune Checkpoint Blockade and the Abscopal Effect: A Critical Review on Timing, Dose and Fractionation. Front Oncol 2018;8:612. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004;64:7985-94. [Crossref] [PubMed]
- Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [Crossref] [PubMed]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [Crossref] [PubMed]
- Welsh JW, Menon H, Tang C, et al. Randomized phase I/II trial of pembrolizumab with and without radiotherapy for metastatic non-small cell lung cancer. J Clin Oncol 2019;37:abstr 9104.
- Minniti G, Anzellini D, Reverberi C, et al. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: evaluation of brain control and toxicity. J Immunother Cancer 2019;7:102. [Crossref] [PubMed]
- Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. [Crossref] [PubMed]
- Deutsch E, Chargari C, Galluzzi L, et al. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol 2019;20:e452-63. [Crossref] [PubMed]
- Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324-33. [Crossref] [PubMed]
- Withers HR, Suwinski R. Radiation dose response for subclinical metastases. Semin Radiat Oncol 1998;8:224-8. [Crossref] [PubMed]
- Zhang X, Niedermann G. Abscopal Effects With Hypofractionated Schedules Extending Into the Effector Phase of the Tumor-Specific T-Cell Response. Int J Radiat Oncol Biol Phys 2018;101:63-73. [Crossref] [PubMed]
- Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res 2018;24:5058-71. [Crossref] [PubMed]
- Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084-91. [Crossref] [PubMed]
- Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol 2012;1:149-54. [Crossref] [PubMed]
- Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31:140-4. [Crossref] [PubMed]
- Venkatesulu BP, Mallick S, Lin SH, et al. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42-51. [Crossref] [PubMed]
- Contreras JA, Lin AJ, Weiner A, et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol 2018;128:498-504. [Crossref] [PubMed]
- Fang P, Jiang W, Davuluri R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol 2018;128:584-90. [Crossref] [PubMed]
- Ladbury CJ, Rusthoven CG, Camidge DR, et al. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiat Oncol Biol Phys 2019;105:346-55. [Crossref] [PubMed]
- Markovsky E, Budhu S, Samstein RM, et al. An Antitumor Immune Response Is Evoked by Partial-Volume Single-Dose Radiation in 2 Murine Models. Int J Radiat Oncol Biol Phys 2019;103:697-708. [Crossref] [PubMed]
- Luke JJ, Lemons JM, Karrison TG, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol 2018;36:1611-8. [Crossref] [PubMed]
- Formenti SC, Demaria S. Understanding Responses to Stereotactic Body Radiotherapy and Pembrolizumab. J Clin Oncol 2018;36:2661-2. [Crossref] [PubMed]
- Meng X, Feng R, Yang L, et al. The Role of Radiation Oncology in Immuno-Oncology. Oncologist 2019;24:S42-S52. [Crossref] [PubMed]
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. [Crossref] [PubMed]
- Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. [Crossref] [PubMed]
- Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51. [Crossref] [PubMed]
- Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 2011;474:216-9. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol 2019;5:1411-20. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Gettinger SN, Wurtz A, Goldberg SB, et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:831-9. [Crossref] [PubMed]
- O'Donnell JS, Smyth MJ, Teng MW. Acquired resistance to anti-PD1 therapy: checkmate to checkpoint blockade? Genome Med 2016;8:111. [Crossref] [PubMed]
- Anagnostou V, Smith KN, Forde PM, et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov 2017;7:264-76. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016;354:1160-5. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Darragh LB, Oweida AJ, Karam SD. Overcoming Resistance to Combination Radiation-Immunotherapy: A Focus on Contributing Pathways Within the Tumor Microenvironment. Front Immunol 2019;9:3154. [Crossref] [PubMed]
- Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Teng F, Meng X, Kong L, et al. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: A systematic review. Cancer Lett 2018;414:166-73. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 2018;19:1180-91. [Crossref] [PubMed]
- Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med 2018;24:144-53. [Crossref] [PubMed]
- Grassberger C, Ellsworth SG, Wilks MQ, et al. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol 2019;16:729-45. [Crossref] [PubMed]
- Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer 2019;19:558. [Crossref] [PubMed]
- Hwang WL, Pike LRG, Royce TJ, et al. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol 2018;15:477-94. [Crossref] [PubMed]
- Hwang WL, Niemierko A, Hwang KL, et al. Clinical Outcomes in Patients With Metastatic Lung Cancer Treated With PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy. JAMA Oncol 2018;4:253-5. [Crossref] [PubMed]
- Bang A, Wilhite TJ, Pike LRG, et al. Multicenter Evaluation of the Tolerability of Combined Treatment With PD-1 and CTLA-4 Immune Checkpoint Inhibitors and Palliative Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;98:344-51. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Vansteenkiste JF, Naidoo J, Faivre-Finn C, et al. Efficacy of durvalumab in patients with stage III NSCLC who experience pneumonitis (PACIFIC). Ann Oncol 2019;30:v592-3. [Crossref]
(English Language Editor: B. Madden)


