Novel ALK mutation with durable response to brigatinib—a case report
Introduction
Rearrangements in the anaplastic lymphoma kinase (ALK) gene occur in about 6–13% of non-small cell lung cancer (NSCLC) (1). When present, ALK tyrosine kinase inhibitors are the clear standard of care for these tumors, based on improvements in response rate, progression-free survival and tolerability compared to cytotoxic chemotherapy (2). While responses with newer, next-generation ALK TKIs are increasingly durable, most patients eventually develop acquired resistance, often mediated by point mutations within the ALK kinase solvent front (3). Investigation into how specific acquired mutations predict sensitivity to ALK kinase inhibitors is ongoing, but much of the available evidence is preclinical. We present the following case in accordance with the CARE Guideline. Here, we discuss an ALK L1196Q mutation emerging during treatment with alectinib for a NSCLC harboring an EML4-ALK fusion that then responded to third-line brigatinib therapy. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-145).
Patient information
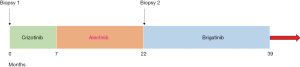
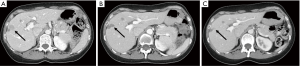
A 60-year-old female presented in November 2016 with cough and right sided flank pain. She was a never smoker. Diagnostic CT imaging revealed a primary left lower lobe lung mass, pathologically enlarged mediastinal and hilar lymphadenopathy and multiple liver metastases. A CT guided liver biopsy confirmed stage IV lung adenocarcinoma and molecular testing identified an EML4-ALK fusion. She was treated with crizotinib 250 mg twice daily and achieved a partial response that lasted 7 months. Scans then showed progression in the liver and new brain metastases. She began second line alectinib 600 mg twice daily and achieved a rapid response in both the liver and brain metastases which was maintained for 15 months. A routine CT scan then revealed a new liver metastasis; all other disease remained unchanged and there were no new symptoms or findings on exam. A positron emission tomography (PET) scan revealed fluorodeoxyglucose (FDG) activity in the new liver lesion with no PET-avid disease in the other areas. Circulating tumor DNA (ctDNA) testing showed only the original EML4-ALK fusion with no new ALK mutations. Biopsy of the new liver lesion confirmed NSCLC and NGS identified the known EML4-ALK fusion as well as an acquired ALK L1196Q mutation. She began third-line brigatinib 90 mg daily, escalating to 180 mg daily in November 2018. A repeat PET/CT in December 2018 showed resolution of FDG uptake in the new liver metastasis with no change in any other sites of disease (Figure 1). Serial imaging of brain and body has shown ongoing disease control now 17 months after starting third line brigatinib (Figure 2). She has been tolerating treatment well without any untoward side effects.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the subject for publication of this case report.
Discussion
ALK kinase inhibitors are the standard initial therapy for NSCLC harboring an ALK fusion and provide rapid, deep and durable responses. While crizotinib was the first agent approved in this setting, next-generation ALK inhibitors such as alectinib and brigatinib have further improved first-line outcomes (4,5). Resistance to next-generation ALK inhibitors such as alectinib is complex and strategies to overcome and prevent resistance are under investigation. Other ALK kinase inhibitors have shown activity, and lorlatinib is currently approved for use after progression on alectinib.
At this time, use of subsequent ALK inhibitors is empiric, but detection of specific, acquired ALK point mutations through NGS is increasingly feasible. Acquired ALK mutations are present in about 20% of patients following first-line crizotinib but detected in more than half of patients who progress on first-line alectinib or ceritinib (6-8). Existing data predicting sensitivity to ALK inhibitors based on the acquired mutation profile could potentially guide therapeutic decisions but is largely preclinical (8-10).
The ALK gatekeeper mutation L1196M is one of the more commonly described resistance mutations (11). Brigatinib is expected to maintain activity in the presence of the ALK L1196M mutation based on preclinical models (12). The ALK L1196Q mutation encountered in this case has not yet been described clinically but would be expected to have similar properties to L1196M. A preclinical report has described ALK 1196Q-mediated resistance to both alectinib and crizotinib (13). While use of brigatinib was, in this case, successful, it is a single case which remains a major limitation in interpretation.
Conclusions
This report is the first clinical description of an ALK L1196Q mutation emerging on alectinib followed by successful and durable treatment with brigatinib. This case also highlights the potential for false-negative ALK mutation results when only plasma is used, particularly when progression is not widespread. In this case, tissue biopsy and molecular testing was required to reveal the mechanism of resistance—and care was taken that the biopsy was of the new liver lesion and not one of the responding lesions, which would not have offered useful clinical information. Use of specific ALK resistance mutations to guide therapy is rational but not yet clinically validated. Fortunately, this very approach is the focus of the ALK Master Protocol: an ongoing prospective, cooperative group trial (NCT 03737994) which will hopefully shed more light on the increasingly relevant field of ALK kinase inhibitor resistance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-145
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-145). SVL reports grants from Alkermes, Bayer, Blueprint, Corvus, Merus, Molecular Partners, Rain Therapeutics, RAPT, Spectrum, Turning Point Therapeutics, grants, personal fees and non-financial support from AstraZeneca, Genentech/Roche, Merck/MSD, non-financial support from Boehringer-Ingelheim, grants and personal fees from Bristol-Myers Squibb, Pfizer, personal fees from Catalyst, Celgene, G1 Therapeutics, Guardant Health, Janssen, Lilly, LOXO, PharmaMar, Regeneron, Takeda outside the submitted work. SVL serves as an unpaid editorial board member of Translational Lung Cancer Research from Jan 2020 to Dec 2021. HL has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the subject for publication of this case report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Choi SH, Kim DH, Choi YJ, et al. Multiple receptor tyrosine kinase activation related to ALK inhibitor resistance in lung cancer cells with ALK rearrangement. Oncotarget 2017;8:58771-80. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol 2013;31:1105-11. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Okada K, Araki M, Sakashita T, et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine 2019;41:105-19. [Crossref] [PubMed]