
The KEYNOTE-189 trial as a new paradigm making cure a reality for metastatic non-squamous non-small cell lung cancer
Lung adenocarcinoma accounts for approximately 60% of all cases of non-small cell lung cancer (NSCLC). With the progression of genomic medicine, precision oncology has contributed to improve survival outcomes and quality of life in NSCLC patients. Besides molecular targeted therapy, immunotherapies are also becoming potential treatment options for advanced NSCLC patients. Pembrolizumab (brand name Keytruda) is a humanized antibody which has been approved in many advanced cancers as a potent immunotherapy. It binds to the programmed cell death protein 1 (PD-1) receptor and blocks its interaction with PD-L1 and PD-L2, which helps restore the immune response. In particular, the lung cancer research in the KEYNOTE-189 trial has achieved excellent results.
The KEYNOTE-189 study (1) is a randomized double-blind phase 3 trial with epidermal growth factor receptor (EGFR)- or anaplastic lymphoma kinase (ALK)-negative metastatic NSCLC patients who received no previous treatment. Patients were randomly assigned in a 2:1 ratio to receive pemetrexed and a platinum-based drug plus either 200 mg of pembrolizumab or placebo every 3 weeks for 4 cycles. Subsequent maintenance therapy with either pembrolizumab or placebo combined with pemetrexed was performed until the end of 35 cycles. Crossover to pembrolizumab monotherapy was permitted among the patients in the placebo-combination group who had verified disease progression. The primary end points were overall survival (OS) and progression-free survival (PFS). According to the latest data released at the 2020 American Society of Clinical Oncology Annual Meeting (2), as of May 20, 2019, the median follow-up was 18.8 (range, 0.2–38.8) months. Addition of pembrolizumab to pemetrexed-platinum reduced the risk of disease progression by 51% versus standard of care chemotherapy (9.0 vs. 4.9 months). Pembrolizumab plus pemetrexed-platinum resulted in a significant OS benefit over the placebo plus pemetrexed-platinum (22.0 vs. 10.6 months, HR =0.56; 95% CI, 0.46–0.69), despite 40.8% patients in the placebo group being cross-treated with pembrolizumab. In particular, the improvement of survival benefit of pembrolizumab combined with chemotherapy occurred independently of PD-L1 expression and a PD-L1 negative population. The results of the safety analysis showed a similar incidence of grade 3–5 adverse events (AEs) in the pembrolizumab and placebo groups, at 72.1% and 66.8%, respectively. The incidence rates of grade 3–5 immune-mediated AEs and infusion-related reactions were a bit higher for the pembrolizumab group compared to the placebo group (12.1% vs. 4.5%). The most common immune-mediated AEs in the pembrolizumab group were hypothyroidism, followed by pneumonia and hyperthyroidism. At the American Association for Cancer Research (AACR) 2019 Annual Meeting, it was reported that combined immunochemotherapy with pembrolizumab led to an OS and PFS benefit in patients with liver or brain metastases and in patients without metastases, compared to chemotherapy alone (3). Given the excellent survival outcomes and tolerable toxicities of the KEYNOTE-189 trail, pembrolizumab plus pemetrexed-platinum was therefore approved as first-line treatment for metastatic non-squamous NSCLC in the USA, Europe, Japan, and China.
Before the advent of immunotherapy, chemotherapy combined with bevacizumab was the standard first-line treatment for advanced non-squamous NSCLC. The BEYOND (4) and E4599 (5) trials were two randomized studies that evaluated the efficacy and safety of bevacizumab combined chemotherapy versus chemotherapy alone as first-line treatment in advanced non-squamous NSCLC. Both studies showed that the objective response rate (ORR), PFS, and OS were significantly better in the bevacizumab plus chemotherapy group compared to the chemotherapy alone group. However, the improvement of OS for bevacizumab combined chemotherapy was less when compared to pembrolizumab combined chemotherapy (BEYOND, OS HR =0.68; E4599, OS HR =0.79; KEYNOTE-189, OS HR =0.56). Pembrolizumab reduced the risk of death by 12–23% more than bevacizumab, indicating that the long-term survival benefit of immunotherapy is more apparent in advanced non-squamous NSCLC.
Furthermore, in advanced non-squamous NSCLC, PD-L1 inhibitor atezolizumab plus chemotherapy with (IMpower150) or without bevacizumab (IMpower130) is also a first-line option. In the IMpower130 (6) study, the addition of atezolizumab to carboplatin plus nab-paclitaxel significantly improved PFS (7.0 vs. 5.5 months, HR =0.64, 95% CI, 0.54–0.77, P<0.0001) and OS (18.6 vs. 13.9 months, HR =0.79, 95% CI, 0.64–0.98, P=0.033) compared to chemotherapy alone. However, the magnitude of OS benefit in reduction of the risk of death in the IMpower130 study was only 21%, but was 44% in the KEYNOTE-189 study. In addition, in the subgroup analysis of IMpower130, there was no significant survival benefit for patients with liver metastasis and or in the PD-L1 subgroup including those with PD-L1-low expression. However, in the KEYNOTE-189 study, the OS HR was always better than that of the IMpower130 study, regardless of the state of PD-L1 expression and liver or brain metastasis, indicating greater survival benefit. In the IMpower150 (7) study, there was improvements of PFS (8.3 vs. 6.8 months; HR =0.62; 95% CI, P<0.001) and OS (19.2 vs. 14.7 months; HR =0.78; 95% CI, 0.64 to 0.96; P=0.02) in the atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP) group compared to the bevacizumab plus carboplatin plus paclitaxel (BCP) group. Notably, patients with liver metastases and those with EGFR or ALK mutations also derived benefit from quadruplet therapy. However, it should note that the short-term OS benefit was not remarkable since the survival curves of ABCP and BCP intertwined within 6 months. In addition, similar to the IMpower130 study, the patients with low or negative PD-L1 expression did not gain a significant OS benefit in the IMpower150. In this respect, the KEYNOTE-189 regimen, in which all patients could significantly benefit from pembrolizumab regardless of PD-L1 expression, has an advantage compared to other first-line immuno-oncology trials.
In summary, irrespective of PD-L1 expression, pembrolizumab combined with pemetrexed and platinum showed significantly better response and survival compared to chemotherapy alone while also not incurring a marked increase in immune-mediated AEs (Table 1). Despite the emergence of new treatment modalities, such as those in the CheckMate-227 (8), CheckMate-9LA (9) and CameL (10) trials, the KEYNOTE-189 regimen is still the internationally recognized standard for first-line metastatic non-squamous EGFR/ALK-negative NSCLC in terms of response rate and long-term survival benefit.
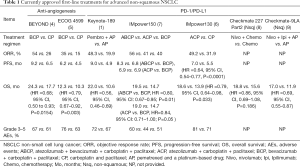
Full table
Acknowledgments
Funding: The present study was funded by the National Natural Science Foundation of China (no. 81772464).
Footnote
Provenance and Peer Review: This article was a free submission. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-874). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Garassino MC, Gadgeel S, Esteban E, et al. Outcomes among patients with metastatic nonsquamous NSCLC with liver metastases or brain metastases treated with pembrolizumab plus pemetrexed-platinum: results from the KEYNOTE-189 study. Cancer Res 2019. [Crossref]
- Zhou C, Wu YL, Chen G, et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2197-204. [Crossref] [PubMed]
- Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010;5:1416-23. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Paz-Ares L, Ciuleanu TE, Yu X, et al. LBA3 Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (aNSCLC): CheckMate 227 part 2 final analysis. Presented at: 2019 ESMO Immuno-Oncology Congress; December 10-14, 2019;30:XI67-8.
- Reck M, Ciuleanu TE, Cobo Dols M, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 2020;38:abstr 9501.
- Zhou C, Chen G, Huang Y, et al. OA04.03 A Randomized Phase 3 Study of Camrelizumab plus Chemotherapy as 1st Line Therapy for Advanced/Metastatic Non-Squamous Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:S215-6. [Crossref]
(English Language Editor: J. Gray)


