
A narrative review of toxicity of chemoradiation and immunotherapy for unresectable, locally advanced non-small cell lung cancer
Introduction
Despite declining tobacco smoking rates (1), lung cancer remains the second most common malignancy in the United States and the leading cause of cancer-related mortality (2). Non-small cell lung cancer (NSCLC) comprises roughly 85% of cases, and patients tend to present with advanced disease (2). Locally advanced (stage III) NSCLC is a broad, widely heterogeneous category encompassing small tumors with mediastinal nodal involvement (T1a–T2bN2+); moderately sized tumors with hilar to mediastinal nodal involvement (T3N1+); and large, invasive, or multiple (ipsilateral) lobe disease of any N stage (T4N0+) (3). About 1/3rd of patients with NSCLC present with locally-advanced disease and these patients are deemed unresectable due to various factors including bulky nodal disease, multi-station lymphadenopathy, extensive mediastinal, airway or other organ invasion, and/or pre-existing medical comorbidities. Historically, standard of care in inoperable patients was chemoradiation (CRT) with concurrent platinum doublet chemotherapy, which resulted in a 5-year overall survival of ~15% (4). Given these poor outcomes, there has been significant interest in improving outcomes through treatment intensification, either through radiation dose escalation or molecularly-targeted therapies. The recently published long term update from the randomized, phase III RTOG 0617 trial demonstrated one of the highest reported 5-year overall survival (OS) figures from any phase III trial in stage III NSCLC at 32.1% in the standard dose CRT arms (with or without experimental cetuximab) (5). However, efforts to improve outcomes through radiation therapy (RT) dose escalation with standard fractionation to 74 Gray (Gy), or the addition of cetuximab on that trial were unsuccessful (5,6). In fact, radiation dose escalation to 74 Gy had a detrimental effect on OS that was attributed in part to increased cardiac dose and esophageal toxicity.
It is also hypothesized that prolonged overall treatment time (OTT) may have contributed to the failure to improve OS, lending interest to hypofractionated or hyperfractionated regimens that shorten overall treatment duration (7,8). A single arm prospective trial found that hyperfractionated radiation therapy with sequential chemotherapy yielded survival rates comparable to outcomes with concurrent chemoradiation (9). Another randomized prospective trial without chemotherapy found an OS benefit with hyperfractionation compared to conventionally fractionated radiation therapy, without chemotherapy (10). Despite this, hyperfractionation studies with concurrent chemoradiation have not consistently found a significant OS benefit with respect to standard fractionation (7,11). A systematic analysis investigating hypofractionation in stage III NSCLC found no significant correlation between 1-, 2-, and 3-year OS and acute effects BED lesional dose (BED10), but reported an absolute OS benefit of 0.36–0.7% for every 1 Gy increase in BED (8). Thus, dose escalation through hypofractionation or SBRT boost is an active area of investigation.
In the setting of persistently poor survival outcomes and failed radiation dose intensification, there is significant interest in adding immunotherapy to the treatment of stage III NSCLC. In 2011, ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitor, became the first immune checkpoint blockade (ICB) therapy to receive FDA approval with an indication in metastatic melanoma (12). Shortly after FDA approval of ipilimumab, several ICB agents targeting the programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway received FDA approval in a variety of cancers, including metastatic NSCLC (12). In 2018, the randomized, phase III PACIFIC trial first established the role for consolidative immune checkpoint blockade (ICB) therapy with the PD-L1 inhibitor durvalumab after CRT in stage III NSCLC by demonstrating significantly improved (progression free survival) PFS and OS with respect to CRT alone (13,14). While very promising, these findings have generated questions regarding the most effective immune pathways to target, appropriate sequencing of ICB therapy, the most effective radiation techniques, and toxicity-related concerns. This review will highlight recent and ongoing prospective trials in unresectable, locally advanced NSCLC that incorporate chemotherapy, radiation, and immunotherapy with an emphasis placed on analysis of treatment-related toxicities. Some of the information used to write this review was collected from PUBMED (date of last search 5 August 2020) using combinations of search terms including “chemotherapy”, “radiation”, “immunotherapy”, “lung cancer”, “unresectable,” and “stage III”. On occasion, reference lists of identified articles were searched manually. Several studies, not yet published, were identified through presentations at major international research meetings, and these abstracts were collected using online search functions. Only works published in the English language were included.
We present the following review in accordance with the Narrative review checklist (available at http://dx.doi.org/10.21037/tlcr-20-638).
Toxicity of chemoradiation without immunotherapy
Prior to combined modality therapy, nearly 40 years ago, RTOG 7301 established 60 Gy as the optimal RT dose in Stage III NSCLC (15). In the 1990s, multiple trials demonstrated that sequential chemotherapy followed by RT improved OS with respect to RT alone (16,17) with later studies demonstrating that concurrent CRT was superior to sequential therapy at the expense of increased esophageal toxicity (4,18). After phase I and phase II data suggested that radiation dose escalation up to 74 Gy was safe and associated with an OS benefit, the phase III RTOG 0617 trial (NCT00533949) enrolled patients in a 2×2 randomization scheme to standard dose (SD) (60 Gy) versus high dose (HD) (74 Gy) RT with concurrent carboplatin (AUC 2) and paclitaxel (45 mg/m2) and to concurrent and maintenance cetuximab (400 mg/m2 loading dose followed by 250 mg/m2 weekly) versus no further therapy (5,6). Two weeks after CRT, all patients received two cycles of consolidation chemotherapy three weeks apart with carboplatin (AUC 6) and paclitaxel (200 mg/m2).
A total of 544 patients were enrolled, of which 424 were evaluable for the radiation dose endpoints proposed (207 patients HD group, 217 patients SD group). 85% and 88% of patients in the HD (arms B and D) and SD (arms A and C) groups respectively completed concurrent chemotherapy per protocol. RT treatment delays were more common with HD radiation (17.6% vs. 11.7%). After a planned futility analysis, randomization to HD therapy closed early. There were 9 grade (G) 5 adverse events possibly attributed to treatment in the HD cohort and 8 in the SD arms but no significant difference in overall rate of G3+ toxicity (79.7% HD vs. 76.6% SD, P=0.44). Despite significantly higher mean lung dose and lung volume receiving 20 or more Gy (lung V20) with HD radiation, there was no difference in G3+ pulmonary toxicity (20.6% SD vs. 19.3% HD). Rates of G3+ radiation pneumonitis were 4% and 7% for HD and SD radiation, respectively. However, esophageal dose was significantly higher with HD treatment leading to increased rates of G3+ dysphagia and/or esophagitis (20.8% vs. 7.3%, P<0.0001). Heart V5 and V30 were also significantly higher with HD therapy. 5-year OS and PFS were substantially inferior with HD treatment at 18.3% and 13% versus 32.1% and 23.1% with SD, respectively, and on multivariate analysis, cardiac dose (V5/V30) and esophageal toxicity were significantly associated with inferior OS. Specifically, increasing RT planning treatment volume (PTV) (HR =1.323, P=0.0219), G3+ esophagitis/dysphagia (HR =1.540, P=0.0079), and heart V5 (HR =1.008, P=0.0051) were independently associated with inferior OS. Both conformal radiation and intensity modulated RT (IMRT) were allowed on the trial, and a planned secondary analysis found that although IMRT reduced toxicity including G3+ pneumonitis rates as well as lower heart doses, this difference did not translate to an observed difference in OS between modalities (19). Taken together, although RTOG 0617 did not show a benefit to radiation dose escalation, it set a new standard for OS with standard dose CRT and suggested the importance of minimizing radiation dose and subsequent treatment-related esophageal and cardiac toxicities due to detrimental effects on survival.
A multi-institution, phase I, dose finding trial (NCT0241237) investigated the safety of adding the poly ADP-ribose polymerase (PARP) inhibitor veliparib to standard of care CRT (20). A total of 48 patients received doses of veliparib ranging from 60 to 240 mg twice a day (BID) during CRT with standard doses from the SD arm of RTOG 0617. Patients then received either 120 or 240 mg of veliparib BID during 2 cycles of consolidation chemotherapy with carboplatin AUC 6 and paclitaxel 200 mg/m2. Toxicity data has been presented at ASCO 2019, and showed that dose limiting influenza and pneumonia occurred in 1 out of 8 patients receiving 200 mg BID/120 mg BID veliparib. Dose limiting toxicities (including esophagitis) occurred in 2 out of 5 patients who received the max dose of 240 mg BID veliparib. Results from a separate multi-institution, placebo-controlled, randomized phase II trial comparing CRT outcomes with or without veliparib in unresectable Stage III NSCLC were also presented in abstract form at ASCO 2019 (NCT01386385) (21). Despite an accrual goal of 132 patients, the trial closed after accruing only 31 evaluable patients after data from the PACIFIC trial became available. These patients received concurrent CRT and consolidative chemotherapy with dosing as per RTOG 0617 with randomization to either 120 mg BID veliparib during CRT and 80 mg veliparib on days 1–7 of consolidative chemotherapy or placebo. Overall, there were 6 acute G3 toxic events in each arm during CRT and 2 and 3 G4 events with veliparib versus placebo, respectively. Rates of acute G3 pneumonitis and esophagitis were 0% and 6% with veliparib and 8% vs. 0% with placebo, respectively. Overall, rates of single digit pulmonary and esophageal toxicity in this phase II trial compare favorably to the SD arms from RTOG 0617, although longer follow up is needed.
In the early 2000s, a phase III trial (SWOG 0023, NCT00020709) was conducted evaluating the benefit of maintenance gefitinib, an epidermal growth factor (EGFR) inhibitor, to standard of care concurrent CRT in patients with unknown EGFR mutation status (22). A total of 243 patients were accrued before an unplanned interim analysis found that the gefitinib arm had significantly worse OS, and the study was thus terminated. Patients were treated with cisplatin (50 mg/m2) and etoposide (50 mg/m2) chemotherapy concurrent with RT to 61 Gy in 1.8–2.0 Gy fractions followed by 3 cycles of docetaxel, and patients with no evidence of progression were randomized to maintenance gefitinib 250 mg/day or placebo. During concurrent RT, a 13% rate of G3+ esophagitis was reported. Additionally, a 7% rate of G3+ pneumonitis was noted after definitive chemoradiation including a 1% rate of fatal pneumonitis. The results from this trial led to the premature closure of an additional phase II trial (NCT00040794) evaluating gefitinib concurrent with RT in patients unselected for EGFR mutation status (23). A total of 63 patients were enrolled before trial closure. They received two cycles carboplatin (AUC 6) and paclitaxel (200 mg/m2) plus gefitinib 250 mg daily. Patients deemed to be “poor risk” by performance status 2 and/or presence of greater than or equal to 5% weight loss then received 66 Gy in 33 fractions with concurrent gefitinib, and patients who did not meet those criteria were considered “good risk” and received concurrent carboplatin (AUC 2) and paclitaxel (50 mg/m2) in addition to RT and gefitinib at the same dosing. All patients continued on consolidation gefitinib at unchanged dose until disease progression. There were 19% and 31% reported rates of G3+ esophagitis in the “poor” and “good” risk groups, respectively and 15% and 16% rates of pneumonitis or pulmonary infiltrates including one lethal pulmonary event in each arm.
Whether protons can further improve outcomes compared to photon based chemoradiation by sparing more normal tissue (lungs, heart, bone marrow) is also a significant topic of interest to the field of radiation oncology. A randomized trial conducted at MD Anderson comparing proton-based with photon (IMRT)-based CRT for locally-advanced NSCLC consisted of 149 patients (IMRT 92 patients, proton 57 patients) with primary endpoints of G3+ radiation pneumonitis and local failure (NCT00915005) (24). G3+ radiation pneumonitis rates were 6.5% for IMRT photon v 10.5% for protons and local failure was very similar (10.5–10.9%). Interestingly, protons decreased lung doses only at certain dose levels 5–10 Gy (RBE), but exposed less heart tissue at all dose levels between 5–80 Gy (RBE). Despite this, the study failed to show that protons improved the primary endpoints. Conversely, a recent propensity-weighted comparison of proton CRT vs. photon CRT for locally-advanced cancers was presented at ASCO 2019, and showed that protons were associated with reductions in 90 day G2+ and G3+ adverse events, and lower decline in performance status (25). Additionally, a single institution analysis reported 16% and 0% rates of G3 and G4 acute leukopenia, respectively, with proton therapy for stage III NSCLC (26) which compared favorably to the 25% and 3% rates of G3 and G4 acute leukopenia in the 60 Gy arm without cetuximab on RTOG 0617 (6). If proton therapy can minimize reductions in white blood cell counts, it may increase the effectiveness of the immune response associated with concurrent or consolidative ICB, as lymphopenia around the time of immunotherapy delivery has been associated with impaired PFS and OS in locally advanced or metastatic NSCLC (27). RTOG 1308 (NCT01993810) is a large phase III, randomized trial that is currently accruing and comparing proton-based CRT to conventional photon-based CRT in the treatment of stage II–IIIB NSCLC. This trial will provide more data to address this question. Patients will be randomized to 35 fractions of photon versus proton based RT with concurrent carboplatin/paclitaxel or cisplatin/etoposide followed by consolidation therapy. Primary endpoints are OS and rates of cardiac toxicity and lymphopenia. Expected enrollment is about 330 patients.
Consolidative ICB in unresectable stage III NSCLC
The practice-changing PACIFIC trial (13,14) has recently evaluated the benefit of adding consolidative ICB targeting the PD-1/PD-L1 axis in unresectable Stage III NSCLC after CRT. The PD-1 pathway is thought to primarily regulate peripheral immune activity (12) by providing feedback inhibition in response to immune activity. T-cells express PD-1, and PD-1 interaction with PD-L1 and PD-L2 provides a negative costimulatory signal that diminishes T-cell activation. Thus, anti PD-1 pathway drugs boost immune response by blocking inhibition of T-cell activation. The randomized, phase III PACIFIC trial enrolled 713 patients with unresectable stage III NSCLC who did not have disease progression after 2 or more cycles of platinum-based CRT. Patients were randomized in a 2:1 ratio to consolidation therapy with the anti-PD-L1 antibody durvalumab (10 mg/kg IV every 2 weeks) or placebo for 12 months with the first dose delivered 1 to 42 days after completion of CRT (13,14). Durvalumab significantly increased 2-year OS (66.3% vs. 55.6% with placebo, P=0.0025) and median PFS (17.2 vs. 5.6 months, HR for disease progression or death, 0.51, 95% CI: 0.41–0.68) (14).
Overall, durvalumab therapy was reasonably well tolerated and 49.0% of patients completed durvalumab therapy as planned, with disease progression being the most common reason for discontinuation (61.4%). With regards to toxicity-related discontinuation, 15.4% and 9.8% of patients discontinued therapy in the durvalumab and placebo arms, respectively, and pneumonitis and pneumonia were the most common reasons for discontinuation of therapy. Similar rates of G3–4 and G5 adverse events of any cause were observed between arms, 30.5% and 4.4% with durvalumab and 26.1% and 6.0% with placebo, respectively (13). In the durvalumab arm, G5 toxicities were predominately cardiopulmonary including 4 cases of pneumonitis and 1 radiation pneumonitis event. The initial publication reported immune-mediated toxicity rates of 24.2% and 8.1% in patients receiving durvalumab and placebo, respectively, resulting in higher rates of glucocorticoid use with durvalumab (13). Pneumonitis of any grade including radiation pneumonitis was reported in 32.8% and 23.5% of patients receiving durvalumab and placebo (14). Rates of G3–4 pneumonitis were more similar at 3.4% for durvalumab and 2.1% with placebo. Subgroup analysis of the trial found that patients who developed pneumonitis were more likely to be Asian (47.9% vs. 17.6%) or have EGFR mutations (11.0% vs. 3.8%) but that durvalumab did not increase pneumonitis in these groups (28). Taken together, consolidative durvalumab offered significant benefit to OS and PFS at the expense of modestly increased but largely manageable risk of immune-mediated toxicities, including pneumonitis.
Until recently, PACIFIC was the only noteworthy publication evaluating chemoradiation and ICB therapy, but multiple prospective trials have recently provided additional published data, and others have reported initial findings in abstract form at major international conferences. Similarly to durvalumab on the PACIFIC trial, the Hoosier Cancer Research Network performed a single arm, multi-institution phase II trial, LUN 14-179 (NCT02343952), assessing the benefit of consolidative therapy with the PD-1 inhibitor pembrolizumab (200 mg IV every 3 weeks for 1 year) after chemoradiation in patients who had no evidence of disease progression 4-8 weeks after CRT (29,30). A total of 93 patients were enrolled to a variety of allowable chemotherapy regimens. The median number of completed cycles of pembrolizumab was 13.5 with 37% of patients completing a full year of immunotherapy (29). Rates of G2+ and G3+ pneumonitis were 17.2% and 5.4% with 1 death attributed to pneumonitis (29). Patient clinical, biologic, radiographic, and dosimetric data was retrospectively reviewed to identify factors associated with development of pneumonitis (31). Of the variables of interest, only right lung V5 achieved significance, although a trend towards significance was noted in patients with pre-existing interstitial lung disease. Total lung V5, 10, 15, 20, 25, and 30 did not predict pneumonitis. Of note, dosimetric, radiographic, and biologic data were only available for a subset of patients. In this limited sample, LUN 14–179 demonstrated modest rates of low grade and fatal pneumonitis that were comparable to the PACIFIC trial providing corroborating evidence for the safety of consolidative ICB with anti PD-1/PD-L1 agents in Stage III NSCLC. Table 1 provides a summary of this and other recent trials involving ICB in unresectable Stage III NSCLC.
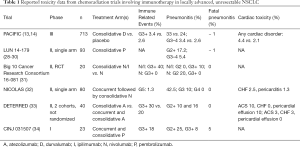
Full table
In addition to targeting the PD-1/PD-L1 axis for consolidative ICB, there is emerging interest in CTLA4 inhibition in unresectable Stage III NSCLC. Two signals, T-cell receptor activation and CD28 costimulatory binding by B7 ligand, are necessary to mount a T-cell response. CTLA4 competes with CD28 for binding by B7 and represses T-cell activation. Because CTLA4 has a higher binding affinity for B7 than CD28 does, upregulation of CTLA4 after T-cell receptor activation prevents B7 binding to CD28, thereby blocking the second signal required for T-cell activation. As a result, anti-CTLA4 antibodies (such as ipilimumab) help to upregulate a T-cell anti-tumor immune response. Given the promising results with consolidative ICB targeting the PD-1/PD-L1 axis, a recent randomized, multi-center, phase II trial in the Big Ten Cancer Research Consortium (16-081) evaluated the benefit of CRT with consolidative nivolumab (PD-1 inhibitor) vs. combined nivolumab/ipilimumab after CRT in patients with unresectable Stage III NSCLC (NCT03285321) (32). The results have not been published, but interim safety data was presented at ASCO 2019 (32). At that time, 20 of a planned 105 patients were evaluable (10 per arm). Rates of total G3–4 adverse events were similar between arms with no treatment-related deaths; there were 4 G3 and 1 G4 events with nivolumab alone and 3 G3 and 1 G4 events with combination nivolumab/ipilimumab. Rates of G2 and G3–4 pneumonitis were 20% and 0% in the nivolumab arm and 10% and 10% in the nivolumab/ipilimumab arm, respectively. There was also a 10% rate of G3 colitis, pancreatitis, and asymptomatic amylase elevation in the nivolumab/ipilimumab arm with none in the nivolumab arm. As expected, the rate of G3 immune related toxicity was slightly higher in the dual immunotherapy arm. The study continues to accrue, and greater enrollment and longer follow up are needed to evaluate the safety and efficacy of dual agent consolidative immunotherapy.
Concurrent ICB in unresectable stage III NSCLC
Given the exciting results of PACIFIC, there is enthusiasm for testing of ICB therapy concurrently with chemoradiation, in the hopes that tumor antigen release during chemoradiation would prime the immune system earlier, leading to improved immunogenic cell death and clinical outcomes. However, there is substantial concern about this approach given the risk of increased toxicity with concurrent ICB. Recently, the European Thoracic Oncology Platform (ETOP) launched the single arm, phase II NICOLAS trial to evaluate the safety and efficacy of concurrent nivolumab and CRT in this patient population (NCT02434081) (33). In this study, concurrent CRT involved 66 Gy in 33 fractions, 3 cycles of platinum chemotherapy, and nivolumab at 360 mg every 3 weeks followed by consolidative nivolumab at 480 mg every 4 weeks. The primary study endpoint was G3+ pneumonitis within 6 months of CRT completion. Data from 80 evaluable patients was available at time of publication of an initial safety evaluation (33). The 46.3% of patients completed one year of immunotherapy. There was a 6.5% and 1.3% rate of Grade 3+ esophagitis and dysphagia, respectively, which was comparable to the SD arm on RTOG 0617 (5). The rate of any, G3, and G4–5 pneumonitis were 42.5%, 10% and 0%, respectively (33). This G3+ pneumonitis rate of 10% is slightly elevated compared to RTOG 0617, where rates of G3+ pneumonitis ranged from 0–4% depending on the arm. There was no association between lung radiation dose, either mean or V20, and increased risk of pneumonitis. Seven fatalities (9%) were observed; 1 death secondary to autoimmune disorder was attributed to immunotherapy, 1 esophageal fistula was considered due to standard CRT, and 1 bronchopulmonary hemorrhage which was likely disease-related (but also possibly related to RT).
Similarly to the NICOLAS trial, MD Anderson conducted a single arm, single institution, phase II trial testing the benefit of atezolizumab (anti PD-L1) in both the consolidative and concurrent settings (DETERRED, NCT02525757) (34). In part 1, 10 patients received conventional CRT, 60–66 Gy in 30–33 fractions, with carboplatin and paclitaxel. If there was no evidence of progression 3 weeks after CRT, these patients received 2 cycles of consolidative chemotherapy with carboplatin and paclitaxel plus atezolizumab (1,200 mg IV once per cycle) followed by maintenance atezolizumab therapy for up to 1 year. In part 2 of the trial, 30 patients received chemoradiation concurrent with atezolizumab followed by the same consolidative and maintenance therapy as in part 1. In the consolidative immunotherapy only cohort (part 1), median PFS and OS were 18.6 and 22.8 months after a median follow up of 22.5 months. With a median follow up period of 15.1 months in the concurrent ICB cohort (part 2), median PFS was 13.2 months and median OS was not reached. Both part 1 and part 2 had 80% rates of grade 3+ adverse events of any kind which the authors related to toxicity from the 2 cycles of full dose consolidative chemotherapy with atezolizumab. Treatment discontinuation occurred in 30% of patients in part 2, 20% related to toxicity. One patient in the consolidative treatment only cohort (part 1) died from a tracheoesophageal fistula (10%). In the concurrent ICB cohort, there were 3 total deaths (10%) all of which were considered non-cancer-related: neutropenic sepsis, gastric hemorrhage, and acute MI. There were no immune-related grade 5 toxicities. There were 20% and 30% rates of grade 3+ immune-related toxicity events with concurrent and consolidative only ICB groups, respectively, including 16% and 10% rates of grade 2+ pneumonitis. There was only one G3 pneumonitis in the concurrent ICB group (3%), and no grade 4–5 pneumonitis observed in either part 1 or part 2. G3+ esophagitis was not present in either cohort. Overall, the authors felt that the treatment regimen is relatively safe and feasible.
CINJ 031507 is a trial evaluating the benefit of adding concurrent ICB to CRT in stage II–IIIB NSCLC (NCT02621398) (35). Patients in this multi-institution, phase I, dose escalation study received standard CRT with weekly carboplatin/paclitaxel plus consolidative pembrolizumab (anti-PD-1) every 3 weeks starting 2–6 weeks after CRT for up to 18 total cycles in dose level 1. In subsequent dose levels, pembrolizumab was moved up to start 2 weeks before end of CRT (dose levels 2 and 3), and then during the start of CRT (dose levels 4 and 5), followed by a safety expansion cohort with a dose of 200 mg pembrolizumab every 3 weeks beginning with the start of radiation. A total of 23 patients were enrolled, of which 21 received at least 1 cycle of pembrolizumab, with 9 patients at dose level 5 and expansion cohort. Primary endpoints were maximum tolerated dose and dose limiting toxicity. Dose limiting toxicity was not observed. There was an 18% rate of grade 3+ immune related adverse events, and 24%, 5%, and 5% rates of grade 2, 3, and 5 pneumonitis, respectively. Pneumonitis did not correlate with lung V2.5, V5, V2, mean lung, or mean heart dose. In summary, the authors also deemed this treatment approach promising and relatively well tolerated.
Ongoing trials in unresectable stage III NSCLC
KEYNOTE-799 (NCT03631784) is a multi-arm phase II study evaluating the safety and efficacy of concurrent and consolidative pembrolizumab ICB in stage III NSCLC (36) that will provide additional data investigating this topic previously only addressed by smaller phase I and II trials (CINJ 031507, ETOP NICOLAS, and DETERRED) (33-35). All patients will receive up to 17 cycles of pembrolizumab (200 mg IV every 3 weeks). Full dose chemotherapy with either carboplatin/paclitaxel or cisplatin/pemetrexed will be delivered with pembrolizumab in cycle 1, and standard CRT to 60 Gy in 30 fractions with reduced dose chemotherapy and pembrolizumab will be administered with cycles 2 and 3. The primary end points are rates of G3+ pneumonitis and rate of complete or partial treatment response. Target accrual is roughly 216 patients. Table 2 summarizes this and other ongoing trials in unresectable stage III NSCLC.

Full table
To date, the benefit of concurrent ICB with CRT in these patients has only been tested in phase I and II trials (33,34), but 2 randomized, multi-institution, phase III trials addressing this question are currently accruing. PACIFIC-2 (NCT03519971) will compare concurrent and maintenance durvalamab to historical standard of care CRT without any immunotherapy (37). Patients will be randomized in a 2:1 ratio to either durvalumab (1,500 mg IV every 4 weeks) concurrent with CRT followed by maintenance durvalumab or placebo concurrent with CRT followed by consolidative placebo therapy. Primary endpoints are PFS and objective response rate. Expected enrollment is around 300 patients. In contrast to PACIFIC-2, the control arm in ECOG EA5181 (NCT04092283) will be the experimental arm from the PACIFIC trial, while the experimental arm will also include concurrent durvalumab. Patients will be randomized to CRT with concurrent and consolidative durvalumab or to CRT followed by consolidative durvalumab alone. Patients randomized to receive concurrent durvalumab will receive it on days 1 and 15 of cycle 1 and day 1 of cycle 2 of chemotherapy. Acceptable chemotherapy regimens include cisplatin/etoposide, cisplatin/pemetrexed, and carboplatin/paclitaxel. All patients will receive consolidative ICB therapy to be delivered within 14 days of completion of radiation with additional cycles every 28 days for 12 cycles. The primary endpoint is OS with a target enrollment of 660 patients.
Although data for consolidative ICB has been previously reported (13,14,29,32), the value of induction ICB prior to CRT to further prime the immune system remains largely uninvestigated. AFT-16 (NCT03102242) is a single arm, phase II, multi-institution, pilot study evaluating the benefit of neoadjuvant ICB in unresectable stage III NSCLC (38). Patients will receive 4 cycles of induction atezolizumab at 1,200 mg IV every 21 days with restaging after cycles 2 and cycle 4 before CRT with 60 Gy and 30 fractions and weekly carboplatin AUC 2 and paclitaxel 50 mg/m2. Patients with disease progression after cycle 2 will proceed to CRT if still indicated as curative therapy. CRT will be followed by consolidation chemotherapy with carboplatin AUC 6 and paclitaxel 200 mg/m2 for 2 cycles beginning 3–5 weeks after radiation and then adjuvant atezolizumab every 21 days for up to a total of 1 year after the start of induction therapy. Expected enrollment is around 63 patients, and the primary endpoint is disease control rate after 12 weeks of induction ICB.
In the era of immunotherapy, it remains unknown whether conventionally fractionated, photon-based radiotherapy remains the most effective way to deliver RT, and how much benefit chemotherapy adds to priming an immune system response. NRG LU004 (NCT03801902) is a randomized, multi-institution, phase I study comparing the safety and efficacy of durvalumab with hypofractionated radiotherapy or conventional radiotherapy in patients with stage II–III NSCLC. Patients will be randomized to hypofractionated radiation in 15 daily fractions or conventional RT over 30 fractions. Patients will not receive chemotherapy, and all patients will receive IV durvalumab every 4 weeks for 13 cycles starting 2 weeks prior to RT. The primary endpoint is rate of toxic events, and estimated enrollment is 24 patients.
As indications for immunotherapy have expanded from metastatic to stage III NSCLC, trials are now investigating the use of targeted therapies currently indicated for Stage IV disease for definitive management of locally advanced disease. LAURA (NCT03521154) is a phase III, randomized, multi-center trial for patients with either exon 19 deletion or L858R EGFR-mutated, unresectable, Stage III disease (39). In a 2:1 ratio, patients will be randomized to consolidative therapy with the third generation, EGFR tyrosine kinase inhibitor osimertinib (80 or 40 mg daily) versus placebo to begin within 6 weeks of completion of standard CRT. The primary endpoint is PFS, and expected enrollment is 200 patients.
Conclusions
After the PACIFIC trial demonstrated a PFS and OS benefit to maintenance anti PD-L1 therapy after concurrent chemoradiation, standard of care shifted to include consolidative ICB (durvalumab) for unresectable stage III NSCLC, especially for patients with PD-L1 expression 1% or greater. The benefit of durvalumab largely seems to outweigh the added risks of toxicity with ICB and has been well-accepted as a new standard. One recent approach has been intensification of consolidative ICB therapy. Overall, addition of single agent consolidative ICB (like PACFIC) leads to acceptably higher rates of mild to moderate immune related toxicity with respect to chemoradiation alone. Rates of more serious G3–4 pneumonitis of 0–5% have been noted with single agent (14,29,34) and certainly appear higher at about 10% with dual agent therapy (32), but overall are similar to the 7% rate of grade 3+ radiation pneumonitis seen with standard dose chemoradiation on RTOG 0617 (5). These side effects are generally manageable through corticosteroids and/or discontinuation of therapy (13), but a roughly 1% rate of fatal pneumonitis (G5) has been observed in several studies (14,29). Of note, rates of completion of the full course of ICB in studies with consolidative therapy alone are modest, ranging from 37–49% (14,29).
In terms of concurrent ICB with chemoradiation, a number of phase I/II studies have been published, suggesting the relative safety of the combination, but appear to have slightly increased rates of mild to moderate immune related toxicity with respect to consolidative ICB alone. A 18–20% rate of grade 3+ immune-related toxicity events was seen with concurrent and consolidative ICB on the CINJ 031507 and DETERRED trials (34,35) which was higher than the 3.4% rate seen at the time of initial reporting of the PACIFIC trial (13). Additionally, reported rates of any pneumonitis, ranging from 23–42.5% (33,34) with concurrent and consolidative ICB may be slightly higher than the 10–33% reported rate of pneumonitis with consolidative ICB alone (14,29,32,34). However, rates of grade 3+ pneumonitis of 3–10% (33,34) were relatively similar to 0–5% rates with single agent consolidative ICB alone (14,29,34) and 7% with SD CRT on RTOG 0617 (5). The 10% rate of G3+pneumonitis and 5% G5 (one patient) pneumonitis observed in the NICOLAS and CINJ 031507 trials respectively, however, warrants closer evaluation in ongoing phase II/III trials.
Another important consideration with concurrent ICB is additive esophageal or cardiac toxicity, because acute grade 3+ esophageal toxicity (20.8% HD radiation vs. 7.3% SD radiation) and increased cardiac V5 were linked to inferior OS in the RTOG 0617 radiation dose escalation trial (5,6). Rates of esophagitis in trials with concurrent ICB have ranged from 0–7.8% (33,34) compared to 7.3% on the SD arms of RTOG 0617, suggesting that addition of concurrent immunotherapy is safe with regards to esophageal toxicity. However, cardiac dosimetric data from these trials is limited and with the exception of the PACIFIC trial, thorough analysis of cardiac risk is limited by small sample size and short length of follow up. Because these concurrent ICB trials have shown 4–6% rates of cardiac toxicity including 2.5–3% rates of heart failure (33,34), it is recommended to minimize cardiac dose as much as possible. Additional follow up and accrual to larger, randomized trials is needed particularly to evaluate late toxicities. Several phase II/III trials evaluating concurrent ICB are currently accruing (EA5181, PACIFIC-2, and KEYNOTE 799) and will provide more data on acute and long-term safety. In addition, the safety of neoadjuvant ICB (prior to chemoradiation) needs to be further evaluated in AFT-16 and other studies. Furthermore, reductions in toxicity may be achieved with proton therapy in stage III NSCLC, which is being evaluated in ongoing studies (RTOG 1308, NCT01993810). Finally, a major area of unmet need is the development of early biomarkers (e.g., germline SNPs, blood-based, tissue-based, or imaging based biomarkers) that predict for not only standard toxicities from CRT, but also immune-related toxicities in this new era of immunotherapy. Such biomarkers will enable personalization of therapy, by reducing risk of severe toxicities resulting from ICB, particularly for those patients who would not derive much benefit from ICB based on other biomarkers predicting lack of tumor response to ICB (e.g., PD-L1 expression, tumor mutation burden, etc.).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-638
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-638). The authors have no conflicts of interest to declare.
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-638
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jamal A, King BA, Neff LJ, et al. Current Cigarette Smoking Among Adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep 2016;65:1205-11. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non-Small-Cell Lung Cancer. J CLIN ONCOL 2010;28:2181-90. [Crossref] [PubMed]
- Bradley JD, Hu C, Komaki RR, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38:706-14. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Zehentmayr F, Grambozov B, Kaiser J, et al. Radiation dose escalation with modified fractionation schedules for locally advanced NSCLC: A systematic review. Thoracic Cancer 2020;11:1375-85. [Crossref] [PubMed]
- Kaster TS, Yaremko B, Palma DA, et al. Radical-intent hypofractionated radiotherapy for locally advanced non-small-cell lung cancer: a systematic review of the literature. Clin Lung Cancer 2015;16:71-9. [Crossref] [PubMed]
- van Baardwijk A, Wanders S, Boersma L, et al. Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 2010;28:1380-6. [Crossref] [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. Radiother Oncol 1999;52:137-48. [Crossref] [PubMed]
- De Ruysscher D, van Baardwijk A, Wanders R, et al. Individualized accelerated isotoxic concurrent chemo-radiotherapy for stage III non-small cell lung cancer: 5-Year results of a prospective study. Radiother Oncol 2019;135:141-6. [Crossref] [PubMed]
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018;8:1069-86. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Perez CA, Stanley K, Rubin P, et al. Patterns of tumor recurrence after definitive irradiation for inoperable non-oat cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 1980;6:987-94. [Crossref] [PubMed]
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940-5. [Crossref] [PubMed]
- Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995;87:198-205. [Crossref] [PubMed]
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Kozono DE, Stinchcombe T, Salama JK, et al. Veliparib (Vel) in combination with chemoradiotherapy (CRT) of carboplatin/paclitaxel (C/P) plus radiation in patients (pts) with stage III non-small cell lung cancer (NSCLC) (M14-360/AFT-07). J Clin Oncol 2019;37:8510. [Crossref]
- Argiris A, Miao J, Cristea MC, et al. A dose-finding study followed by a phase II randomized placebo-controlled trial of chemoradiotherapy (CRT) with or without veliparib in stage III non-small cell lung cancer (NSCLC). J Clin Oncol 2019;37:8523. [Crossref]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III Trial of Maintenance Gefitinib or Placebo After Concurrent Chemoradiotherapy and Docetaxel Consolidation in Inoperable Stage III Non-Small-Cell Lung Cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [Crossref] [PubMed]
- Ready N, Jänne PA, Bogart J, et al. Chemoradiotherapy and Gefitinib in Stage III Non-small Cell Lung Cancer with Epidermal Growth Factor Receptor and KRAS Mutation Analysis: Cancer and Leukemia Group B (CALEB) 30106, a CALGB-Stratified Phase II Trial. J Thorac Oncol 2010;5:1382-90. [Crossref] [PubMed]
- Liao Z, Lee JJ, Komaki R, et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1813-22. [Crossref] [PubMed]
- Baumann BC, Mitra N, Harton JG, et al. Comparative Effectiveness of Proton vs. Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. JAMA Oncol 2020;6:237-46. [Crossref] [PubMed]
- Hoppe BS, Flampouri S, Henderson RH, et al. Proton Therapy With Concurrent Chemotherapy for Non-Small-Cell Lung Cancer: Technique and Early Results. Clinical Lung Cancer 2012;13:352-8. [Crossref] [PubMed]
- Cho Y, Park S, Byun HK, et al. Impact of Treatment-Related Lymphopenia on Immunotherapy for Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2019;105:1065-73. [Crossref] [PubMed]
- Vansteenkiste J, Naidoo J, Faivre-Finn C, et al. PACIFIC Subgroup Analysis: Pneumonitis in Stage III, Unresectable NSCLC Patients Treated with Durvalumab vs. Placebo After CRT. J Thorac Oncol 2018;13:S370-1. [Crossref]
- Durm GA, Althouse SK, Sadiq AA, et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. J Clin Oncol 2018;36:8500. [Crossref]
- Durm G, Althouse S, Sadiq A, et al. ChemoXRT W/ Consolidation Pembrolizumab in Unresectable Stage III NSCLC: Long-Term Survival Update and Analysis of Post-Progression Therapy. J Thorac Oncol 2019;14:S627. [Crossref]
- Ahmed SS, Durm GA, Donatelli J, et al. Potential Predictors of Pembrolizumab Associated Pneumonitis: A Retrospective Review of the HCRN LUN 14-179 Trial: Topic: Medical Oncology. J Thorac Oncol 2017;12:S1552. [Crossref]
- Yan M, Durm GA, Mamdani H, et al. Interim safety analysis of consolidation nivolumab and ipilimumab versus nivolumab alone following concurrent chemoradiation for unresectable stage IIIA/IIIB NSCLC: Big Ten Cancer Research Consortium LUN 16-081. J Clin Oncol 2019;37:8535. [Crossref]
- Peters S, Felip E, Dafni U, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—The ETOP NICOLAS trial. Lung Cancer 2019;133:83-7. [Crossref] [PubMed]
- Lin SH, Lin Y, Yao L, et al. Phase II Trial of Concurrent Atezolizumab With Chemoradiation for Unresectable NSCLC. J Thorac Oncol 2020;15:248-57. [Crossref] [PubMed]
- Jabbour SK, Berman AT, Decker RH, et al. Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial. JAMA Oncol 2020;6:848-55. [Crossref] [PubMed]
- Jabbour SK, Park K, Cohn D, et al. Phase 2 trial of first-line pembrolizumab with platinum doublet chemotherapy and radiotherapy in patients (pts) with unresectable, locally advanced stage III non-small-cell lung cancer (NSCLC): KEYNOTE-799. J Clin Oncol 2019;37:TPS8575. [Crossref]
- Bradley JD, Nishio M, Okamoto I, et al. PACIFIC-2: Phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J Clin Oncol 2019;37:TPS8573. [Crossref]
- Ross HJ, Kozono DE, Urbanic JJ, et al. AFT-16: Phase II trial of atezolizumab before and after definitive chemoradiation (CRT) for unresectable stage III non-small cell lung cancer (NSCLC). J Clin Oncol 2020;38:9045. [Crossref]
- Shun L, Nash T, Saggese M, et al. Osimertinib Maintenance After Definitive Chemoradiation in Patients with Unresectable EGFRm-Positive Stage III NSCLC (LAURA). J Thorac Oncol 2018;13:S497. [Crossref]


