Predicting the prognosis of lung cancer: the evolution of tumor, node and metastasis in the molecular age—challenges and opportunities
Introduction
Obvious as it may seem, it is important that the readers of this article keep in mind that the tumor, node and metastasis (TNM) classification of lung cancer is no more and no less than a system to code the anatomic extent of the disease. Therefore, by definition, the TNM classification does not include other elements that, while they can help improve our capacity to prognosticate the disease for a given patient, are unrelated to the anatomy of the tumor, i.e., parameters from blood analysis, tumor markers, genetic signatures, comorbidity index, environmental factors, etc. Prognostic indexes combining the TNM classification and other non-anatomic parameters are called, by consensus between the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), prognostic groups to differentiate them from the anatomic stage groupings.
The TNM classification of lung cancer is applied to all histopathological subtypes of non-small cell carcinoma, to small cell carcinoma and to typical and atypical carcinoids. It is governed by general rules (1-3) (Table 1) that apply to all malignancies classified with this system, and by site-specific rules applicable to lung cancer exclusively (4). There also are recommendations and requirements issued with the objective to classify tumors in a uniform way when their particular characteristics do not fit in the basic rules (4).

Full table
The three components of the classification have several categories that are defined by different descriptors. For lung cancer, those for the T component are based on tumor size, tumor location and involved structures; those for the N, on the absence, presence and location of lymph node metastasis; and those for the M, on the absence, presence and location of distant metastasis. There are optional descriptors that add information on the local aggressiveness of the tumor (differentiation grade, perineural invasion, vascular invasion and lymphatic permeation) all of which have prognostic relevance (5-8); assess the intensity of the investigation to determine the stage (certainty factor); and assess the residual tumor after therapy (residual tumor).
Origin and evolution of the TNM classification for lung cancer
The TNM classification was developed by Pierre Denoit in a series of articles published from 1943 to 1952. It was soon adopted by the UICC that published brochures covering several anatomical sites, the lung being included in 1966. Two years later, the UICC published the first edition of the TNM Classification of Malignant Tumors and agreements were reached with the AJCC, created in 1959 as the American Joint Committee for Cancer Staging and End Results Reporting, to consult each other to avoid publication of differing classifications. Since then, the UICC and the AJCC have been responsible for updating and revising the TNM classifications of malignant tumors with the participation of national TNM committees of several countries and taking into account the published reports on the topic. The second to sixth editions of the UICC manual on the TNM Classification of Malignant Tumors and the first to sixth editions of the AJCC Staging Manual included classifications for lung cancer that had been informed by a progressively enlarging database initially collected by Mountain, Carr and Anderson, and subsequently managed by Mountain. Their database originally contained a little over 2,000 patients, but it had grown to more than 5,000 by the time the fifth edition of the TNM classification for lung cancer was published in 1997. The sixth edition was published in 2002 with no modifications (9).
While the fifth edition of the classification was being printed, the International Workshop on Intrathoracic Staging took place in London, United Kingdom, in October 1996, sponsored by the International Association for the Study of Lung Cancer (IASLC) (10). At that meeting, in the presence of Dr. Mountain, the limitations of the database that had been used to revise the TNM classification for lung cancer were openly discussed. In essence, it was considered that, while the database consisted of a relatively large number of patients, all of them originated from the United States of America, and, therefore, the staging system could not really be called ‘international’, as it was called at that time; and, although all tumors had clinical and pathological classifications, the majority had been treated surgically. So, the database was thought not to be representative of the international community, as there were no patients from other countries; or of the current clinical practice, as there were no patients treated with other therapies. Therefore, an agreement was reached to issue a worldwide call to build a really international database of lung cancer patients treated by all therapeutic modalities. This required the constitution of an International Staging Committee that was approved and given a small amount of funding, to pump-prime, by the IASLC Board in 1998. Subsequently substantial financial support was secured by an unrestricted grant from Eli-Lilly. Cancer Research And Biostatistics (CRAB), a not-for-profit biosciences statistical center in Seattle, was appointed to collect, manage and analyze the new database. The proprietors and managers of known databases were subsequently summoned to attend a series of preparatory meetings to identify potential contributors to the IASLC international database for the purpose of revising the TNM classification of lung cancer.
The 7th edition of the TNM classification of lung cancer
By 2005, more than 100,000 patients had been registered and more than 80,000 met the established criteria for analysis, the largest database ever collected to revise the TNM classification of lung cancer. All these patients originated in 45 databases of different nature in 20 countries around the world, and had been diagnosed with lung cancer between 1990 and 2000 (11). From 2005 to 2009, the members of the subcommittees for the T, the N, and the M components, and those for stage grouping, validation, small-cell lung cancer, carcinoids, visceral pleura invasion, lymph node map, and non-anatomic prognostic factors analyzed, together with the biostatisticians of CRAB, the specific results, proposed recommendations for changes, and wrote their manuscripts that were eventually published in the Journal of Thoracic Oncology (12-23). All recommendations were accepted by the UICC and the AJCC, and included in the lung cancer chapters of the 7th edition of their respective staging manuals (1,2). In addition, the IASLC became the main provider of evidence to the UICC and the AJCC to revise future editions of the TNM classification of lung cancer and other thoracic malignancies, as pleural mesothelioma and thymic tumors had been incorporated into the IASLC Staging Project in 2008 and 2009, respectively. In 2009, the IASLC published its own staging manual and handbook (3,24).
The most important innovations of the 7th edition were the increased relevance of tumor size; the reconciliation of separate tumor nodules in the same lobe, in another ipsilateral lobe and in the contralateral lung with their observed prognosis; the upstaging of malignant pleural and pericardial effusions and nodules to metastatic disease; the relocation of some TNM groups into a different stage; the separation of intrathoracic and extrathoracic metastases; the validation of the TNM classification for bronchopulmonary carcinoid tumors; the recommendation to use the TNM classification for small-cell lung cancer instead of the dichotomous limited and extensive disease classification; and the international and multidisciplinary agreement of a new pulmonary and mediastinal lymph node map. Visceral pleura invasion was defined by the involvement of its elastic layer, and elastic stains were recommended when visceral pleura invasion was not evident with standard stains. These changes were extensively reviewed from the general (25-34), radiological (35,36), clinical (37-39), therapeutic (40-42) and pathological (43,44) points of view; and they were validated, in total or in part, with the series of many institutions (45-63).
The classification of the 7th edition is very useful to indicate prognosis, which is one of the objectives of the classification. The 3-cm cut-point, that had been the only one to separate tumors according to size, was abandoned in favor of five tumor-size groups separated at 2, 3, 5 and 7 cm cut-points, defining groups of tumors with significantly different prognosis (12). The downstaging of separate tumor nodules in the same lobe from T4 (6th edition) to T3 (7th edition), and in another ipsilateral lobe from M1 (6th edition) to T4 (7th edition), increased the awareness of these nodules, that are usually resected, in contradistinction with the contralateral nodules (M1a in 7th edition) that are rarely resected (12). For the N component, the descriptors were unchanged, but the definition of nodal zones, grouping neighboring nodal stations, emphasized the concept of quantification of nodal disease, as it was evident that the more involved zones, the worse the prognosis. Although this information was not used to modify the present N descriptors because it could not be validated clinically, geographically or by T categories, it is practically useful as it helps refine the postoperative prognosis of patients with nodal disease (13). For the M component, the separation of intrathoracic (M1a) from extrathoracic (M1b) metastasis also helps in assessing prognosis as both groups of metastases have different prognosis, but also reconciles common clinical practice as treatment of malignant pleural and pericardial effusions and nodules had been considered palliative, as with metastatic disease, even when these situations were in the T4 category in the previous editions of the TNM classification (14).
The proposed nodal map was the result of a wide international and multidisciplinary consensus (20). It reconciled the differences between the maps proposed by Mountain and Dresler (64) and the Naruke-Japan Lung Cancer Society (65,66), and introduced important innovations: clear anatomical landmarks for each nodal station, recognizable by the radiologist, the endoscopist and the surgeon; the enlargement of the supraclavicular and subcarinal nodal stations; and the shift of the anatomic midline of the mediastinum to the left paratracheal margin (oncological midline) for the purpose of separating right and left superior and inferior paratracheal lymph nodes (20).
In the new stage grouping, some aggregate TNM combinations moved from one stage to another. Large T2 tumors (T2b N0 M0) were upstaged from stage IB to IIA; T2a N1M0 tumors were downstaged from stage IIB to IIA; and T4 N0-1 M0 tumors were downstaged from stage IIIB to IIIA. The question of how to treat patients with these tumors arose. Were T2b N0 M0 tumors to be treated with adjuvant chemotherapy as the other tumors in stage IIA? The perception was that the changes in classification lead to a change in treatment (41,42), but in principle the answer is that treatment recommendations should derive from properly conducted clinical trials and not from taxonomic changes. The mere change of stage does not provide any evidence on the best treatment. New trials will be necessary to answer this question. In the meantime, the multidisciplinary team will have to decide on the best therapeutic option based on all the available information on the patient, the tumor and the surgical resection.
The application of the TNM classification to small-cell lung cancer provides us with a clear example of its utility in refining prognosis. The traditional limited disease group includes tumors from stages IA to IIIB with a 29% absolute survival difference between them: 5-year postoperative survival rates of 38% and 9%, respectively, with the expected progressive degradation of survival as tumor stage increases (18). This survival difference would be lost if the TNM were not applied to small-cell lung cancer and all tumors were put together in the same category of limited disease.
Towards the 8th edition
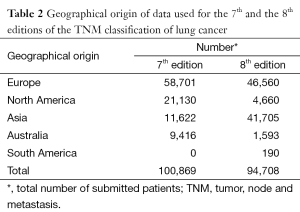
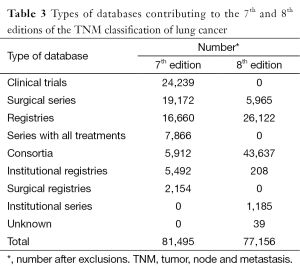
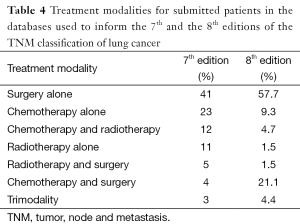
The modifications in the T and the M components of the classification, the recognition of the relevance of the quantification of nodal disease, the new stage groupings, and the application of the TNM classification to small-cell lung cancer improved our capacity to indicate prognosis, but the 7th edition of the TNM classification for lung cancer has limitations derived, mainly, from its retrospective nature (67). Not all databases contained the necessary staging details to validate all descriptors, and over half of the registered patients underwent surgical treatment either alone or in combination (11). This high proportion of surgical cases does not reflect common clinical practice and there is the need of a wider representation in the range of therapeutic modalities. To achieve this, the IASLC made a worldwide call to build a new international database to inform the 8th edition of the TNM classification of lung cancer (68). Amazing as it may seem, the call was answered with the submission of more than 90,000 new patients from 35 databases in 16 countries, diagnosed from 1999 to 2010; and 77,156 (70,967 with non-small cell lung cancer and 6,189 with small-cell lung cancer) met the requirements for analysis (69). Table 2 shows the geographical origin of the data. Europe maintains its leadership in submitting patients, while there was an important drop in contributions from North America and a very relevant increase in cases from Asia, thanks to the massive submission of Japanese registries. Although modest, for the first time there are some patients from South America. Another characteristic of this database is that nearly 4,000 patients were prospectively registered online through the electronic data capture system established by CRAB. These cases have very complete information and have been very useful for certain analyses for which detail matters, such as the number of metastases in patients with M1b disease. Table 3 shows the types of submitted databases. Clinical trials were in the lead in the database used for the 7th edition, while none was submitted for the 8th. The absence of clinical trials and the surgical cases submitted by Japan account for the relative scarcity of advanced cases in the database used for the 8th edition. Table 4 shows the types of treatments for each database. In both, there is a predominance of surgical cases, which is more evident in the database for the 8th edition. This fact may question the generalizability of the recommendations for changes derived from the analyses of the database, as it has been shown that some descriptions, for example tumor size, do not have the same prognostic impact in the populations of patients treated with radiotherapy (70).
Full table
Full table
Full table
At the moment of this writing, the members of the IASLC Staging and Prognostic Factors Committee already have analyzed the database and decided on the changes to be recommended in the 8th edition. The original papers describing these analyses and the recommendations for changes already are submitted to the Journal of Thoracic Oncology or are in the process of being submitted.
Pending the scrutiny from the international oncological community and the acceptance from the UICC and the AJCC, the most important recommended changes affect tumor size, the relevance of which is greater than it was thought from the analyses of the previous database. Consequently, the recommendation is to define more groups of tumors based on size and to include tumor size as a descriptor in all T categories, from Tis to T4. The recommendation for the N component is to retain the 7th edition descriptors, but to propose the quantification of nodal disease by number of involved nodal stations for prospective registration of data. For the M component, the recommendation is to separate extrathoracic single metastasis from multiple metastases, as they have different prognosis. The stage grouping will be slightly modify, as the suggested changes in the T and the M components lead to the creation of more stages both in early and advanced disease. There will also be recommendations to code the new adenocarcinoma subtypes, especially adenocarcinoma in situ and minimally invasive adenocarcinoma; the recommendation to apply the TNM classification to small-cell lung cancer will be emphasized; and an attempt will be made to clarify the classification of lung cancers with multiple lesions: second primary tumors, separate tumor nodules, and multiple nodules with ground glass/lepidic features.
The future of the TNM classification
The TNM classification of lung cancer is the most consistent and solid prognosticator of the disease, but it does not explain the whole prognosis because prognosis is multifactorial. In addition to the anatomic extent of the tumor, patient and environmental factors also count. Prognosis also is dynamic, as it may be different at the time of diagnosis, after treatment or at recurrence (71). In the TNM classification, tumor resection plays an important role as it defines pathological staging and may modify the prognostic assessment based on clinical staging. Other than that, the TNM classification does not include blood analyses, tumor markers, genetic characteristic of the tumor or environmental factors that may account for the differences in survival among similar tumors in different geographic areas.
In order to make progress to indicate a more personalized prognosis, instead of a prognosis based on cohorts of patients with tumors of similar anatomic extent, the IASLC Staging and Prognosis Factors Committee decided to expand its activities to the study of non-anatomic prognostic factors. Therefore, in the third phase of the IASLC Lung Cancer Staging Project, the activities of the committee will be directed to further refine the TNM classification and to find available factors that can be combined with tumor staging to define prognostic groups. To some extent, this already was done with the analyses of the database used for the 7th edition. Prognostic groups with statistically significant differences were defined by combining anatomic tumor extent and very simple clinical variables, such as performance status, gender, and age. These prognostic groups were defined for clinically and pathologically staged tumors, and for small-cell and non-small cell lung cancers (22,23).
The database used for the 8th edition includes several non-anatomical elements related to the patient, the tumor and the environment that may help refine prognosis at clinical and pathological staging (69). Due to the limitations of the previous databases, future revisions of the TNM classification will need to be more balanced in terms of therapeutic modalities, and better populated with patients from underrepresented geographical areas, such as Africa, India, Indonesia, North, Central and South America, and South East Asia. The data contributed in the future will have to be complete regarding the TNM descriptors, and preferably prospective. The more robust the TNM, the more important its contribution to the prognostic groups.
To achieve all of the above, international collaboration is essential. Those interested in participating in this project should send an email expressing their interest to information@crab.org, stating ‘IASLC staging project’ in the subject of the email. The IASLC Staging and Prognostic Factors Committee has been very touched by the overwhelming generosity of colleagues around the world who have contributed cases to inform the 7th and the 8th editions of the TNM classification of lung cancer. We continue to count on their collaboration to further revise future editions and to define prognostic groups that will eventually allow a more personalized indication of prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th ed. Oxford: Wiley-Blackwell, 2009:138-46.
- Edge S, Byrd DR, Compton CC, et al. editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010:253-70.
- Goldstraw P, editor. Staging Manual in Thoracic Oncology. Editorial Rx Press, Orange Park, FL, 2009.
- Wittekind C, Compton CC, Brierley JR, et al. editors. TNM Supplement: A commentary on uniform use. 4th ed. Oxford: Wiley-Blackwell, 2012.
- Poncelet AJ, Cornet J, Coulon C, et al. Intra-tumoral vascular or perineural invasion as prognostic factors for long-term survival in early stage non-small cell lung carcinoma. Eur J Cardiothorac Surg 2008;33:799-804. [PubMed]
- Yilmaz A, Duyar SS, Cakir E, et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur J Cardiothorac Surg 2011;40:664-70. [PubMed]
- Shimada Y, Saji H, Yoshida K, et al. Pathological vascular invasion and tumor differentiation predict cancer recurrence in stage IA non-small-cell lung cancer after complete surgical resection. J Thorac Oncol 2012;7:1263-70. [PubMed]
- Matsumura Y, Hishida T, Shimada Y, et al. Impact of extratumoral lymphatic permeation on postoperative survival of non-small-cell lung cancer patients. J Thorac Oncol 2014;9:337-44. [PubMed]
- Goldstraw P. The history of TNM staging in lung cancer. In: Goldstraw P, editor. Staging manual in thoracic oncology. Orange Park, FL: Editorial Rx Press, 2009:17-29.
- Goldstraw P. Report on the international workshop on intrathoracic staging, London, October 1996. Lung Cancer 1997;18:107-11.
- Goldstraw P, Crowley JJ. The International Association for the Study of Lung Cancer international staging project on lung cancer. J Thorac Oncol 2006;1:281-6.
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol 2007;2:686-93. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:694-705. [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [PubMed]
- Travis WD, Giroux DJ, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2008;3:1213-23. [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90.
- Sculier JP, Chansky K, Crowley JJ, et al. The impact of additional prognostic factors on survival and their relationship with the anatomical extent of disease expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the proposals for the 7th Edition. J Thorac Oncol 2008;3:457-66.
- Chansky K, Sculier JP, Crowley JJ, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009;4:792-801. [PubMed]
- Goldstraw P, editor. Staging Handbook in Thoracic Oncology. Editorial Rx, Orange Park, FL Press, 2009.
- Sculier JP. Diagnostic. The new TNM classification for lung cancer. Rev Mal Respir 2008;25:3S40-7.
- Schneider BJ. Non-small cell lung cancer staging: proposed revisions to the TNM system. Cancer Imaging 2008;8:181-5. [PubMed]
- Detterbeck FC, Tanoue LT, Boffa DJ. Anatomy, biology and concepts, pertaining to lung cancer stage classification. J Thorac Oncol 2009;4:437-43. [PubMed]
- Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther 2009;9:413-23. [PubMed]
- Rami-Porta R, Chansky K, Goldstraw P. Updated lung cancer staging system. Future Oncol 2009;5:1545-53. [PubMed]
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Rami Porta R. New TNM classification for lung cancer. Arch Bronconeumol 2009;45:159-61. [PubMed]
- Goldstraw P. Updated staging system for lung cancer. Surg Oncol Clin N Am 2011;20:655-66. [PubMed]
- Marshall HM, Leong SC, Bowman RV, et al. The science behind the 7th edition Tumour, Node, Metastasis staging system for lung cancer. Respirology 2012;17:247-60.
- Goldstraw P. New TNM classification: achievements and hurdles. Transl Lung Cancer Res 2013;2:264-72. [PubMed]
- Kligerman S, Abbott G. A radiologic review of the new TNM classification for lung cancer. AJR Am J Roentgenol 2010;194:562-73. [PubMed]
- Mirsadraee S, Oswal D, Alizadeh Y, et al. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol 2012;4:128-34. [PubMed]
- Rami-Porta R, Giroux DJ, Goldstraw P. The new TNM classification of lung cancer in practice. Breathe 2011;7:348-60.
- Lyons G, Quadrelli S, Jordan P, et al. Clinical impact of the use of the revised International Association for the Study of Lung Cancer staging system to operable non-small-cell lung cancers. Lung Cancer 2011;74:244-7. [PubMed]
- Goldstraw P. New staging system: how does it affect our practice? J Clin Oncol 2013;31:984-91. [PubMed]
- Rami-Porta R. How new staging affects surgical perspectives. J Thorac Oncol 2009;9:s150-2.
- Boffa DJ, Detterbeck FC, Smith EJ, et al. Should the 7th edition of the lung cancer stage classification system change treatment algorithms in non-small cell lung cancer? J Thorac Oncol 2010;5:1779-83.
- Boffa DJ, Greene FL. Reacting to changes in staging designations in the 7th edition of the AJCC staging manual. Ann Surg Oncol 2011;18:1-3.
- Fisseler-Eckhoff A. New TNM classification of malignant lung tumors 2009 from a pathology perspective. Pathologe 2009;30 Suppl 2:193-9. [PubMed]
- Travis WD, IASLC Staging Committee. Reporting lung cancer pathology specimens. Impact of the anticipated 7th Edition TNM classification based on recommendations of the IASLC Staging Committee. Histopathology 2009;54:3-11.
- Zell JA, Ignatius Ou SH, Ziogas A, et al. Validation of the proposed International Association for the Study of Lung Cancer non-small cell lung cancer staging system revisions for advanced bronchioloalveolar carcinoma using data from the California Cancer Registry. J Thorac Oncol 2007;2:1078-85. [PubMed]
- Ignatius Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM Edition. J Thorac Oncol 2009;4:300-10. [PubMed]
- Ou SH, Zell JA. Validation study of the proposed IASLC staging revisions of the T4 and M non-small cell lung cancer descriptors using data from 23,583 patients in the California Cancer Registry. J Thorac Oncol 2008;3:216-27. [PubMed]
- Oliaro A, Filosso PL, Cavallo A, et al. The significance of intrapulmonary metastasis in non-small cell lung cancer: upstaging or downstaging? A re-appraisal for the next TNM staging system. Eur J Cardiothorac Surg 2008;34:438-43; discussion 443. [PubMed]
- Lee JG, Lee CY, Kim DJ, et al. Non-small cell lung cancer with ipsilateral pulmonary metastases: prognosis analysis and staging assessment. Eur J Cardiothorac Surg 2008;33:480-4. [PubMed]
- Filosso PL, Ruffini E, Pizzato E, et al. Multifocal (MF) T4 non-small cell lung cancer: a subset with favourable prognosis. Interac Cardiovasc Thorac Surg 2008;7:227.
- Lee JG, Lee CY, Bae MK, et al. Validity of International Association for the Study of Lung Cancer proposals for the revision of N descriptors in lung cancer. J Thorac Oncol 2008;3:1421-6. [PubMed]
- Fukui T, Mori S, Hatooka S, et al. Prognostic evaluation based on a new TNM staging system proposed by the International Association for the Study of Lung Cancer for resected non-small cell lung cancers. J Thorac Cardiovasc Surg 2008;136:1343-8. [PubMed]
- Suzuki M, Yoshida S, Tamura H, et al. Applicability of the revised International Association for the Study of Lung Cancer staging system to operable non-small-cell lung cancers. Eur J Cardiothorac Surg 2009;36:1031-6. [PubMed]
- Ruffini E, Filosso PL, Bruna MC, et al. Recommended changes for T and N descriptors proposed by the International Association for the Study of Lung Cancer - Lung Cancer Staging Project: a validation study from a single-centre experience. Eur J Cardiothorac Surg 2009;36:1037-44. [PubMed]
- Kameyama K, Takahashi M, Ohata K, et al. Evaluation of the new TNM staging system proposed by the International Association for the Study of Lung Cancer at a single institution. J Thorac Cardiovasc Surg 2009;137:1180-4. [PubMed]
- Yano T, Morodomi Y, Ito K, et al. Verification of the newly proposed T category (seventh edition of the tumor, node, and metastasis classification) from a clinicopathological viewpoint in non-small cell lung cancer-special reference to tumor size. J Thorac Oncol 2010;5:45-8.
- Strand TE, Rostad H, Wentzel-Larsen T, et al. A population-based evaluation of the seventh edition of the TNM system for lung cancer. Eur Respir J 2010;36:401-7.
- Chien CR, Yang ST, Chen CY, et al. Impact of the new lung cancer staging system for a predominantly advanced-disease patient population. J Thorac Oncol 2010;5:340-3. [PubMed]
- León-Atance P, Moreno-Mata N, González-Aragoneses F, et al. Multicenter analysis of survival and prognostic factors in pathologic stage I non-small-cell lung cancer according to the new 2009 TNM classification. Arch Bronconeumol 2011;47:441-6. [PubMed]
- Haraguchi S, Koizumi K, Akiyama H, et al. Unification of T2a and T2b tumors to T2 tumors in non-small cell lung cancer staging. Ann Thorac Cardiovasc Surg 2011;17:559-64. [PubMed]
- Dassanayake DL, Muthunayake TM, Senevirathna KH, et al. Staging of lung cancer in a tertiary care setting in Sri Lanka, using TNM 7th edition. A comparison against TNM6. BMC Res Notes 2012;5:143.
- Jhun BW, Lee KJ, Jeon K, et al. Clinical applicability of staging small cell lung cancer according to the seventh edition of the TNM staging system. Lung Cancer 2013;81:65-70.
- Wang J, Wu N, Zheng Q, et al. Evaluation of the 7th edition of the TNM classification for lung cancer at a single institution. J Cancer Res Clin Oncol 2014;140:1189-95.
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [PubMed]
- Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg 1978;76:832-9. [PubMed]
- Japan Lung Cancer Society. In: Classification of lung cancer. First English edition. Tokyo: Kanehara and Co Ltd, 2000:38.
- Rami-Porta R, Goldstraw P. Strength and weakness of the new TNM classification for lung cancer. Eur Respir J 2010;36:237-9. [PubMed]
- Giroux DJ, Rami-Porta R, Chansky K, et al. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol 2009;4:679-83. [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Ball D, Mitchell A, Giroux D, et al. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol 2013;8:315-21. [PubMed]
- Brundage MD, Mackillop WJ. Lung Cancer. In: Gospodarowicz MK, O’Sullivan B, Sobin LH, editors. Prognostic Factors in Cancer. 3rd ed. Hoboken: Wiley-Liss, 2006:159-63.


