Small cell lung cancer (SCLC): no treatment advances in recent years
Introduction: is small cell lung cancer (SCLC) research a modern-day Odyssey?
Lung cancer is among the most common tumor types, representing 13% of newly diagnosed cancers worldwide. Both the absolute and relative frequencies of lung cancer have risen dramatically. Unfortunately, it remains by far the leading cause of cancer-related deaths, accounting for 18% of the total number of deaths (1). Approximately 20% of lung tumors exhibit neuroendocrine differentiation, representing a group of neoplasms that share common morphological and immunohistochemical features, including SCLC. SCLC, accounting for 10% of clinical lung cancer cases, is an aggressive malignancy strongly associated with smoking. It displays a distinct natural history characterized by a high growth fraction, rapid doubling time and early establishment of widespread metastatic lesions (2). While 30% of patients present with disease confined to one hemithorax [limited disease (LD)], the majority of cases have disease not encompassed by one radiotherapy field [extended disease (ED)] (3).
Historically, SCLC, previously known as “oat cell carcinoma”, first appeared in the literature in 1936, in an article describing a case of oat cell carcinoma in a patient with asbestosis (4). The lethality of this malignancy was unequivocal, as SCLC rapidly led to fatalities if left untreated (5). Since then, progress has been made in the histopathological diagnosis, staging and treatment of this neoplasm. Early trials have established the role of radiotherapy over surgery as the initial treatment approach (6). However, survival rates were extremely low and chemotherapy emerged as the optimal modality for patients with distant metastatic disease (7). Combination regimens were proven to be superior to single agent chemotherapy (8) with the introduction of alkylator-based chemotherapy regimens in the 70s and cisplatin-based combinations in the 80s. Ultimately, combined chemo-radiotherapy treatment protocols were tested (9) and incorporated into the treatment of LD-SCLC (10). However, since then our knowledge regarding SCLC has entered a dormant state.
In fact, our efforts towards evolution of SCLC therapy resemble the Odyssey: a journey of a constant confrontation with various obstacles, during which the hero alternates between hope and despair until he reaches Ithaca. Apparently, the initial characterization of this disease as chemo- and radio-sensitive, based on the results of clinical trials conducted in the 80s (11,12), led to excessive optimism concerning our ability to cure SCLC. Continuing the metaphor, perhaps this optimism was taken for contempt of the “Olympian Gods” and every effort to evolve SCLC treatment, henceforth, was doomed to fail. Despite promising preclinical data, the results of clinical studies were disheartening.
Indeed, the current standard of care for LD-SCLC disease is concurrent chemoradiation, with four cycles of cisplatin and etoposide with thoracic radiation therapy during the first or second chemotherapy cycle. Resection is indicated for the limited proportion of patients that present with small peripheral primary tumors and no documented infiltration of the mediastinal lymph nodes. Management of patients with ED typically includes four to six chemotherapy cycles with a platinum agent and etoposide or irinotecan. Following a good response to initial therapy, prophylactic cranial irradiation is indicated in patients with LD-SCLC and suggested for patients with ED-SCLC. Despite the fact that first line treatment provides response rates of up to 80%, the prognosis of SCLC remains dismal. The majority of patients relapse within 6 months after completion of initial treatment, leading to a median survival range of 15 to 20 months and eight to 13 months for patients with LD and ED, respectively (13). During the last few decades, although we should acknowledge that efforts to improve the clinical outcome of SCLC patients have been made, the 5-year survival rate remains 6% (14). Numerous treatment strategies have been evaluated, yet none of them has yielded superior outcomes over standard platinum-based therapy (15). It is interesting to note that the last drug that gained approval for treatment of these patients was topotecan in 1998 (16). Based on these sobering data, the absence of real progress is unambiguous, necessitating the vital need for the development of novel strategies in the care of SCLC patients.
The non-small cell lung cancer (NSCLC) research paradigm: a proposed route to “Ithaca”
During the last decade, we have witnessed a revolution in treatment of NSCLC. Significant advances in early detection and therapeutic modalities have led to clinically significant improvements in survival. By employing analogical reasoning, one should expect that SCLC research would have advanced at the same pace. Sadly, however, this is not the case. In order to achieve a deeper understanding of the realities of SCLC we must think critically, taking into account what Odysseus has taught us regarding methods to identify and overcome obstacles and barriers inherent to the nature of SCLC.
Foremost, Odysseus defeated the powerful cyclops Polyphemus when he identified his weakness: having only one eye. Being single-minded regarding SCLC research could be considered to be such an “Achilles heel”, as knowledge gained from other neoplasms could be a useful tool. The paradigm of NSCLC may serve as an archetype in organizing our approach to SCLC, while keeping in mind the unique characteristics of this neoplasm.
As chemotherapy has achieved its potential, progress in NSCLC treatment has been based upon the elucidation of the aberrant molecular pathways involved in the pathogenesis and progression of NSCLC. Thus, the identification of certain mutations in the epidermal growth factor receptor (EGFR) gene or rearrangements of the anaplastic lymphoma kinase (ALK) gene that act as molecular “drivers” of NSCLC have provided new targets for lung cancer treatment. Nowadays, there is no doubt as to the heterogeneity of this neoplasm and pie charts showing the mutational landscape of NSCLC have become standard. The subsequent development of certain targeted therapies has resulted in significantly improved therapeutic efficacy and survival benefit in patients harboring the corresponding driver mutation. Hence, NSCLC has entered the era of personalized cancer management and molecular testing has emerged as an essential component of the decision making process. However, this is not the case for SCLC. Our knowledge regarding the molecular biology of our enemy, although crucial, is minimal. Thus, treatment is only based on the immunohistological identification of SCLC features and is the same for all patients. Certain intrinsic SCLC features serve as limitations in our efforts toward demonstrating its biological heterogeneity.
Finding information in unexpected places
Once again, ‘tissue seems to be the issue’. Typically, the diagnosis of SCLC is based on biopsy (~75%) or cytology samples and not surgical specimens (<2%) (17). As the majority of patients are unresectable at diagnosis, surgery only rarely forms part of the management of these patients. As previously mentioned, resection is indicated in the rare case of a patient presenting with a small peripheral primary lesion (18). It is interesting to note that comprehensive genomic characterization of SCLC is generally based on the analysis of such specimens (17,19,20). However, the biological features of this disease are expected to be different from the typical widespread disease we commonly encounter in clinical practice. As a rule, SCLC patients have multiple comorbid conditions that hamper their ability to undergo surgical biopsy and less invasive procedures are therefore preferred, reducing the amount of tissue that can be obtained for testing. As a result, SCLC is not included in the neoplasms studied by the Cancer Genome Atlas, as it does not meet the inclusion criteria regarding tissue availability.
During his journey, Odysseus had to visit Hades, the world of the dead, to seek the advice of the prophet Tiresias. Helpful travel directions for a safe return to Ithaca came from an unexpected source, a spirit. Likewise, in order to circumvent the need for ample tissue samples, novel approaches in research are being employed. Cell lines, patient-derived xenografts (PDX) and genetically engineered mouse models (GEMM) have been successfully utilized in drug-development preclinical studies (21). Moreover, PDX and GEMM provide a powerful tool in evaluating the intrinsic mechanisms underlying SCLC genesis and proliferation and metastasis in vivo, in a system that closely resembles humans (22-24). Furthermore, circulating tumor cells (CTCs) may serve as a liquid biopsy. Although molecular analysis of CTCs in SCLC is still in its infancy, proof-of-concept studies have shown that it is feasible (25). In fact, the above described tools can be combined as demonstrated in a provocative and innovative effort from Hodgkinson et al. in which CTCs derived from peripheral blood of SCLC patients were used to create mice xenografts [CTC-derived xenografts (CDX)]. Serial blood sampling could enable us to obtain a representative picture of the primary tumor at different treatment phases. Hence, these manipulable models may prove valuable in translational drug discovery and development research.
Similarly, seeking “prophecies” to enhance our understanding of SCLC biology, we should analyze tumor specimens not only from patients that respond to treatment but also from non-responders, or even those who progress after an initial response to treatment. However, due to rapid physical and functional deterioration during disease progression, some SCLC patients are not able to tolerate invasive methods for obtaining tissue samples. Erratic approaches to overcome this limitation suggest biopsying residual disease after completion of first line treatment, when patients are in a better clinical condition, potentially enabling detection of the resistant clone that could lead to disease progression. Furthermore, the development and validation of methods to longitudinally monitor the genomic evolution of the primary tumor (e.g., cell free-DNA) by obtaining blood samples could also prove useful. Whatever the method used, the ability to take serial biopsies with adequate, both in terms of quantity and quality, tumor material from NSCLC patients has been fundamental in elucidating the mechanisms of drug resistance and in developing novel, more potent therapies (26). Prerequisites to achieve the same for SCLC would include optimization of biopsy techniques, introduction of clinical trials that encourage re-biopsy in the setting of disease relapse and reduction of tissue requirements for a more focused genomic analysis (27).
Choose your fights wisely
Since the 90s, it has been widely appreciated that identification of the prevalent genomic alterations in SCLC would reveal the molecular pathways involved in tumor development and provide new therapeutic targets. Pioneering research in this field unveiled the genetic hallmarks of SCLC: inactivation of the tumor suppressor genes TP53 (75–90% of cases) (28,29) and retinoblastoma gene (RB1) (60–90%) (30). However, initial enthusiasm was hampered by the realization that loss-of-function mutations were not the ideal drug targets as restoration of tumor-suppressor function cannot be achieved through conventional drug development strategies (31). Hence, these mutations can be likened to Charybdis and Scylla, two sea-monsters that lived in the opposite sites of a narrow strait. Odysseus and his sailors were caught between two equally deadly alternatives. When they realized that they had no other option but confront the monsters, they continued onward, sailing their ship past Scylla and Charybdis and suffering some fatalities. Similarly, recent efforts have focused on more comprehensive genomic characterization of SCLC in order to move beyond TP53 and RB1 mutations.
It has been shown that tobacco smoke contains over 60 different mutagens (32). Given the indisputable link between tobacco-smoking and SCLC tumorigenesis, a wide variety of diverse genetic defects are expected to form the mutational landscape of this disease. Genomic research has demonstrated the high mutational burden of SCLC in surgical resection specimens, core biopsies and fine-needle aspirations. Thus, frequent genetic alterations apart from TP53 and RB1 mutations include 3p deletions (33), loss of PTEN (34), activating PI3K mutations (35), upregulation of wild-type c-kit (36), MYC amplification (37) and telomerase activation (38). In contrast to NSCLC, EGFR and KRAS mutations are uncommon (28,39) and linked to combined SCLC/adenocarcinoma cases. Currently, studies utilizing genomic and proteomic profiling technology are providing further insights into SCLC biology. Apart from confirming the data from previous studies (40,41), these techniques have brought to light both genetic changes and aberrantly activated signaling pathways that could be considered novel targets for treatment. These include CREBBP, EP300, MLL, SLIT2 and EPHA7 mutations, FGFR1 amplifications (20), RLF-MYCL1 gene-fusion and SOX2 amplification (19), TMEM132D, SPTA1, VPS13B (42) and activating RET mutations (17), BCL2 (40), RICTOR (43) and KIAA1432 gene amplifications (44), intrinsic and autonomous activation of the Hedgehog pathway (45) and finally repair-protein PARP1 and enhancer of zeste homolog 2 (EZH2) overexpression (46).
The heterogeneity of SCLC is now being established. The genetic signature of these tumors may serve as a useful tool to classify lung tumors and identify subgroups of SCLC with distinct biological features. In such a manner, the map of mutations in never-smoker SCLC patients (47) differs from in smokers (48) and these patients would probably benefit from different treatment strategies. Similarly, patients presenting with small peripheral primary tumors might exhibit a different mutational-dependency than patients with widespread metastatic disease.
Nevertheless, the major challenge remains to organize all these chaotic genetic data into something clinically meaningful. It is believed that only a small subset of the numerous somatic mutations observed in SCLC are crucial for tumor cell proliferation and progression. A prerequisite for a genetically-informed approach to SCLC would be identification of these “driver” mutations among the numerous, random or secondary, “passenger” mutations which do not confer a clonal growth advantage to the tumor cells. Furthermore, the paradigm of NSCLC has taught us that driver mutations may be rare genetic defects, found only in a small fraction of tumors. However, pie charts illustrating driver mutations in NSCLC contain data from the analysis of thousands of tissue samples. By contrast, thus far less than 200 SCLC samples have been analyzed using next-generation sequencing in the relative studies. The implementation of bioinformatic approaches to the analysis of a large series of SCLC tissue samples would definitely improve functional characterization of SCLC genomic alterations and pave the way for precision medicine (49-51). Sharing this information on certain websites, such as www.mycancergenome.com would facilitate physicians’ access to genetic data and available clinical trials.
On the other hand, recent data imply that SCLC might be an immunologically manipulable neoplasm. Long-term SCLC survivors are characterized by predominant activity of immune-effector over immune-suppressive mechanisms (52). Indeed, it could be postulated that the high rate of somatic mutations reported in SCLC may contribute to increased immunogenicity. This hypothesis reinforced by reports of improved survival in patients with neurological paraneoplastic syndromes exhibiting anti-Hu immune responses (53) provide the theoretical rationale for evaluation of immunotherapy strategies in the management of SCLC patients.
Focusing interest back on SCLC
SCLC is classed as an “orphan disease”. Over the past years, the attitude of both researchers and the pharmaceutical industry regarding SCLC has been nihilistic. A minor decline in SCLC incidence during the last 30 years (54) together with the recent breakthroughs in translational research in NSCLC may have caused SCLC to be disregarded. This lack of enthusiasm is reflected by the small number of randomized clinical trials in SCLC. A quick search for phase III randomized trials in the published literature reveals more than six times as many NSCLC trials compared to SCLC. Only 125 of 2,499 presentations during the 16th World Conference on Lung Cancer were dedicated to SCLC (55). Since January 2010, approximately 100 interventional phase II and phase III clinical trials have been enrolled in the registry of ClinicalTrials.gov (56), with a shift towards evaluating new agents in ED-SCLC and relapsed disease (57). Considering the low possibility of a positive phase II trial going forward to a successful phase III trial (58), these remarkably low numbers are clearly insufficient.
Scientific interest and research funding has been directed towards NSCLC drug development and SCLC seems to have be forgotten. Lung cancer researchers and the pharmaceutical companies appear to be living in the land of the Lotus-Eaters. Here it was that some of the Odysseus’ sailors ate the lotus. This fruit was so delicious that those who tasted it lost their desire to return home. Odysseus, showing great leadership skills, realized the threat and forced his crew back to the ships and tied them up, readdressing their focus on reaching Ithaca. Similarly, pioneers in SCLC should live up to the challenge posed and lead efforts to facilitate progress in this particular field of oncology. An important step toward this goal is the Scientific Framework for Small Cell Lung Cancer (59), an international initiative created by the National Cancer Institute (NCI) based on the Recalcitrant Cancer Research Act of 2012 (60). Basic, translational and clinical research scientists produced a consensus statement that provides recommendations to guide our efforts in meeting the underlying challenges of SCLC. The five proposed pillars that we should build to provide foundation for fruitful research projects are: (I) optimization of research tools used in preclinical studies of SCLC; (II) growing knowledge about the molecular biology of SCLC; (III) development of novel screening and early detection approaches for high risk populations; (IV) evaluation of therapeutic strategies targeting specific dependencies acquired by SCLC; and (V) elucidation of the biological mechanisms that govern response or resistance to therapy. Alongside highlighting funding opportunities, this scientific framework has drawn attention to SCLC by focusing on research priorities.
Precision medicine and immunotherapy: have we reached the land of the Phaeacians?
Currently, there are no diagnostic approaches that facilitate early detection of SCLC and have a significant impact on patient survival (61). Therefore, the discovery of effective treatments is of paramount importance. Efforts have been made to improve the efficacy of cytotoxic chemotherapy but so far have not improved clinical outcomes (62-66) (Table 1). In fact, it is widely accepted that empiric chemotherapy for SCLC has probably plateaued and further evaluation of chemotherapy variations is probably not warranted.

Full table
Based on recent advances in defining the molecular landscape of SCLC, a shift towards a translational approach to treatment is emerging. Various molecular targeted therapies have already been evaluated in different settings in SCLC. However, to date no agent has been approved as the clinical trials results have, for the most part, been disappointing (67-86) (Table 2).
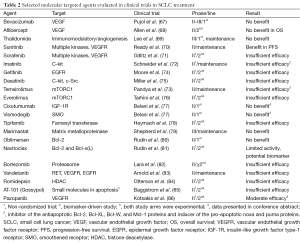
Full table
Digging deeper into the rationale, design and patients enrolled in these trials, it is possible to speculate on the reasons for this failure. Firstly, although in the majority of trials there was a valid theoretical background, with the identification of a single target, the patient population was not selected on the basis of a predictive biomarker. Thus, the targeted therapy was empirically evaluated. Low frequency of the targeted molecular aberration in the study population would therefore negatively influence results. Moreover, driver mutations in this neoplasm have not yet been identified so any trial blocking a secondary molecular pathway with no effect on SCLC oncogenesis would, by definition, be negative. Additionally, considering the high rate of co-existing mutations in SCLC specimens, it is likely that strategies utilizing single agent therapy are unlikely to achieve a clear impact on survival. The substantial clonal heterogeneity observed in SCLC probably necessitates the employment of multi-kinase tyrosine kinase inhibitors or combined targeted therapy strategies to achieve durable and clinically meaningful responses (87). Lastly, most phase II trials conducted were non-randomized, single-arm studies and results were compared to historical data. This process is biased and often gives false signals regarding drug efficacy. Nevertheless, the drug-development process in NSCLC can be useful in guiding us through these challenges. On the one hand, key objectives of phase I trials could also include identification of possible predictive biomarkers in expanded patient cohorts at the maximum tolerated dose. On the other, launching small, randomized phase II trials that employ biomarker enrichment strategies (88) may prove cost-effective, while increasing confidence in the chances of a successful phase III trial.
Therapeutic progress in SCLC is long overdue. Towards accelerating the pace of drug development, pharmacogenomic approaches to define sensitivity to certain drugs have been adopted. The NCI’s Developmental Therapeutics Program is currently using the NCI-60 tumor cell line collection to determine sensitivity to more than 400 targeted agents and 100 FDA-approved oncology treatments in vitro (89,90). This approach has already identified some interesting drugs, such as polo-like kinase (PLK) inhibitors, HSP-90, Aurora kinase, HDAC and PARP inhibitors (21,91,92). Research into predictive biomarkers for these strategies is currently ongoing. Screening existing drugs as part of a drug repositioning, bioinformatic approach might also prove useful since tricyclic antidepressants have recently been shown to exert potent antineoplastic activity ex vivo (93). Thus, integrated pharmacogenomics could prove helpful in prioritizing drug candidates for clinical trial evaluation. However, data derived from these analyses should still be interpreted with caution (94) and understood as a basis for hypothesis.
This is an exciting time in SCLC treatment. As active research in SCLC is in progress, novel therapeutic targets have emerged and multiple molecular targeted agents and immunotherapy strategies are currently being evaluated, with promising results in early studies. For example, the Aurora A kinase inhibitor, alisertib, is currently being evaluated in a randomized phase II trial as second line therapy in combination with paclitaxel (NCT02038647). Preclinical data suggesting MYC amplification as a possible predictive biomarker (91) remain to be confirmed. In a multi-histology, phase II basket trial, alisertib demonstrated an overall response rate of 21% in patients with relapsed SCLC (95).
Similarly, PARP inhibition is supported by a solid preclinical rationale (46,96) and a possible predictor of response (92). Following encouraging initial results from phase I trials (97), several PARP inhibitors (talazoparib, veliparib) are being tested in multiple clinical trials as monotherapy (NCT01286987), in combination with chemotherapy (NCT01638546, NCT01642251) in various settings, and even as maintenance treatment (NCT02289690).
Moreover, SCLC models have been shown to be quite sensitive to cyclin-dependent-kinase 7 (CDK7) inhibition (98). As a result, a phase Ib/II study of roniciclib, a CDK7 inhibitor, in combination with chemotherapy as first-line treatment in patients with ED-SCLC is currently ongoing (NCT01573338).
RET inhibition has also been evaluated, in an empiric manner. Vandetanib failed to prolong survival when used as a maintenance treatment (83) in unselected patients. However, recently a rare activating RET mutation (M918T) has been identified in a metastatic SCLC lesion and cell lines overexpressing the mutant protein were shown to be susceptible to RET inhibition (17). It remains to be seen whether this preclinical observation could be translated into improved clinical outcomes in the small percentage of SCLC patients harboring the specific RET mutation.
In the same manner, mTORC1 inhibitors have failed to improve outcomes in unselected SCLC patients (73,76), though the PI3K-AKT-mTOR pathway remains in the spotlight of drug development. A proof-of-concept phase II trial has been designed in order to evaluate PF-05212384 (PKI-587), a dual PI3K/mTOR inhibitor in pretreated SCLC patients that exhibit features suggestive of PI3K-pathway activation. Recently, an integrated pharmacogenomics study has recognized RICTOR amplification as a predictive biomarker for mTORC1/2 inhibition (99). Biomarker-driven, randomized clinical trials are needed to test this hypothesis.
In addition, a novel class of biopharmaceutical agent is under evaluation in clinical studies. Rovalpituzumab tesirine is an antibody-drug conjugate (ADC) showing promising efficacy in a selected SCLC population. It consists of a Delta-like protein 3 (DLL3) targeted antibody linked to a potent pyrrolobenzodiazepine dimer toxin (100). In a phase Ia/Ib clinical trial the ADC resulted in a 44% overall response rate and 95% clinical benefit rate, demonstrating substantial clinical activity in pretreated patients with DLL3 positive SCLC (101).
Similar to other tumor types, immunotherapy looks set to revolutionize SCLC treatment (102). Although early trials with IFN-a in combination with chemotherapy (103) or vaccines in the adjuvant setting (104) have failed to improve outcomes, successes with immune checkpoint inhibitors in NSCLC have renewed interest in the development of immunologic strategies for SCLC treatment. Early signs indicative of an additive effect of immunotherapy on chemotherapy came from a phase II trial of ipilimumab, an anti-CTLA4 monoclonal antibody, in combination with carboplatin and paclitaxel as first-line treatment in ED-SCLC patients (105). In relapsed disease, anti-PD1 monoclonal antibodies (pembrolizumab, nivolumab) are having an impact with impressive response rates, comparing favorably to historical outcomes (106,107). Nevertheless, questions are being raised whether PDL-1 expression is the ideal biomarker to predict response to anti-PD1 inhibition.
All the above mentioned studies represent a ray of light in the 30-year darkness of failure and struggle against this dismal disease. Perhaps this is the last stop in our journey to Ithaca, as was the friendly, safe land of the Phaeacians for Odysseus. However, the past has taught us that overwhelming optimism may only lead us to failure once again. Only time will tell whether targeted therapy and immunotherapy can improve clinical outcomes to such a degree that they constitute a meaningful change in SCLC treatment.
Conclusions
In spite of recent advances in elucidating the aberrant molecular pathways that dictate SCLC oncogenesis, this malignancy remains an important public health problem, leading to the death of approximately 16,000 patients per year in the United States (14). For decades, cytotoxic chemotherapy has remained the backbone of treatment but, while SCLC is a chemo-sensitive disease, experience shows that high response rates are not universally translated into a cure. Nevertheless, it is high time progress was made in SCLC research and we have all the necessary tools at our disposal. Every failure is a lesson learnt, every success a battle fought. Our aim must be to improve the prognosis of patients with SCLC.
We are still on the long journey to Ithaca and should not let the Sirens of excessive optimism distract us from our goal. With determination and cunning we can reach Ithaca. By any means, whatever the final result may be, the entire research process will make us wiser.
“As you set out for Ithaca, hope the voyage is a long one, full of adventure, full of discovery……And if you find her poor, Ithaca won’t have fooled you. Wise as you will have become, so full of experience, you will have understood by then what these Ithakas mean” (The Canon by CP Cavafy, translated by Edmund Keeley and Philip Sherrard).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [PubMed]
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer 1995;75:191-202. [PubMed]
- Gloyme SR. A case of oat-cell carcinoma of the lung occurring in asbestosis. Tubercule 1936;18:100-1.
- Kato Y, Ferguson TB, Bennett DE, et al. Oat cell carcinoma of the lung. A review of 138 cases. Cancer 1969;23:517-24. [PubMed]
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 1973;2:63-5. [PubMed]
- Green RA, Humphrey E, Close H, et al. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516-25. [PubMed]
- Lowenbraun S, Bartolucci A, Smalley RV, et al. The superiority of combination chemotherapy over single agent chemotherapy in small cell lung carcinoma. Cancer 1979;44:406-13. [PubMed]
- Livingston RB, Moore TN, Heilbrun L, et al. Small-cell carcinoma of the lung: combined chemotherapy and radiation: a Southwest Oncology Group study. Ann Intern Med 1978;88:194-9. [PubMed]
- Osterlind K, Hansen HH, Hansen HS, et al. Chemotherapy versus chemotherapy plus irradiation in limited small cell lung cancer. Results of a controlled trial with 5 years follow-up. Br J Cancer 1986;54:7-17. [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [PubMed]
- Jänne PA, Freidlin B, Saxman S, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 2002;95:1528-38. [PubMed]
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015;4:533-44. [PubMed]
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 1997;15:2090-6. [PubMed]
- Dabir S, Babakoohi S, Kluge A, et al. RET mutation and expression in small-cell lung cancer. J Thorac Oncol 2014;9:1316-23. [PubMed]
- Quoix E, Fraser R, Wolkove N, et al. Small cell lung cancer presenting as a solitary pulmonary nodule. Cancer 1990;66:577-82. [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [PubMed]
- Wildey G, Chen Y, Lent I, et al. Pharmacogenomic approach to identify drug sensitivity in small-cell lung cancer. PLoS One 2014;9:e106784. [PubMed]
- Calbo J, van Montfort E, Proost N, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011;19:244-56. [PubMed]
- Kwak I, Tsai SY, DeMayo FJ. Genetically engineered mouse models for lung cancer. Annu Rev Physiol 2004;66:647-63. [PubMed]
- Daniel VC, Marchionni L, Hierman JS, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res 2009;69:3364-73. [PubMed]
- Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Popper HH, Tímár J, Ryska A, et al. Minimal requirements for the molecular testing of lung cancer. Transl Lung Cancer Res 2014;3:301-4. [PubMed]
- Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol 2001;28:3-13. [PubMed]
- Takahashi T, Takahashi T, Suzuki H, et al. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene 1991;6:1775-8. [PubMed]
- Mori N, Yokota J, Akiyama T, et al. Variable mutations of the RB gene in small-cell lung carcinoma. Oncogene 1990;5:1713-7. [PubMed]
- Wiman KG. Pharmacological reactivation of mutant p53: from protein structure to the cancer patient. Oncogene 2010;29:4245-52. [PubMed]
- Pfeifer GP, Denissenko MF, Olivier M, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21:7435-51. [PubMed]
- Petersen I, Langreck H, Wolf G, et al. Small-cell lung cancer is characterized by a high incidence of deletions on chromosomes 3p, 4q, 5q, 10q, 13q and 17p. Br J Cancer 1997;75:79-86. [PubMed]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 1998;17:475-9. [PubMed]
- Shibata T, Kokubu A, Tsuta K, et al. Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett 2009;283:203-11. [PubMed]
- Tamborini E, Bonadiman L, Negri T, et al. Detection of overexpressed and phosphorylated wild-type kit receptor in surgical specimens of small cell lung cancer. Clin Cancer Res 2004;10:8214-9. [PubMed]
- Noguchi M, Hirohashi S, Hara F, et al. Heterogenous amplification of myc family oncogenes in small cell lung carcinoma. Cancer 1990;66:2053-8. [PubMed]
- Sarvesvaran J, Going JJ, Milroy R, et al. Is small cell lung cancer the perfect target for anti-telomerase treatment? Carcinogenesis 1999;20:1649-51. [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [PubMed]
- Kim YH, Girard L, Giacomini CP, et al. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene 2006;25:130-8. [PubMed]
- Voortman J, Lee JH, Killian JK, et al. Array comparative genomic hybridization-based characterization of genetic alterations in pulmonary neuroendocrine tumors. Proc Natl Acad Sci U S A 2010;107:13040-5. [PubMed]
- Iwakawa R, Kohno T, Totoki Y, et al. Expression and clinical significance of genes frequently mutated in small cell lung cancers defined by whole exome/RNA sequencing. Carcinogenesis 2015;36:616-21. [PubMed]
- Ross JS, Wang K, Elkadi OR, et al. Next-generation sequencing reveals frequent consistent genomic alterations in small cell undifferentiated lung cancer. J Clin Pathol 2014;67:772-6. [PubMed]
- Iwakawa R, Takenaka M, Kohno T, et al. Genome-wide identification of genes with amplification and/or fusion in small cell lung cancer. Genes Chromosomes Cancer 2013;52:802-16. [PubMed]
- Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med 2011;17:1504-8. [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [PubMed]
- Sun JM, Choi YL, Ji JH, et al. Small-cell lung cancer detection in never-smokers: clinical characteristics and multigene mutation profiling using targeted next-generation sequencing. Ann Oncol 2015;26:161-6. [PubMed]
- Pleasance ED, Stephens PJ, O'Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184-90. [PubMed]
- Hou JP, Ma J. DawnRank: discovering personalized driver genes in cancer. Genome Med 2014;6:56. [PubMed]
- Akavia UD, Litvin O, Kim J, et al. An integrated approach to uncover drivers of cancer. Cell 2010;143:1005-17. [PubMed]
- Bashashati A, Haffari G, Ding J, et al. DriverNet: uncovering the impact of somatic driver mutations on transcriptional networks in cancer. Genome Biol 2012;13:R124. [PubMed]
- Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res 2008;14:6770-9. [PubMed]
- Graus F, Dalmou J, Reñé R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol 1997;15:2866-72. [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [PubMed]
- 16th World Conference on Lung Cancer. IASCL-International Association for the Study of Lung Cancer, Sep 6-9, 2015. [Cited: Sep 29, 2015]. Available online: http://library.iaslc.org/virtual-library-search?product_id=1&author=&category=Small+Cell+Lung+Cancer
- ClinicalTrials.gov. National Library of Medicine (NLM) at the National Institutes of Health (NIH). [Cited: Sep 29, 2015]. Available online: https://clinicaltrials.gov/ct2/home
- Malik SM, Korn E, Thomas A, et al. Small cell lung cancer: Why has it become an orphan disease? J Clin Oncol 2015;33:abstr 7578.
- Maitland ML, Hudoba C, Snider KL, et al. Analysis of the yield of phase II combination therapy trials in medical oncology. Clin Cancer Res 2010;16:5296-302. [PubMed]
- National Cancer Institute. Scientific Framework for Small Cell Lung Cancer (SCLC). [Cited: Sep 29, 2015]. Available online: http://deainfo.nci.nih.gov/advisory/ctac/workgroup/SCLC/SCLC%20Congressional%20Response.pdf
- H.R.733 - Recalcitrant Cancer Research Act of 2012. [Cited: Sep 29, 2015]. Available online: https://www.congress.gov/bill/112th-congress/house-bill/733
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Satouchi M, Kotani Y, Shibata T, et al. Phase III study comparing amrubicin plus cisplatin with irinotecan plus cisplatin in the treatment of extensive-disease small-cell lung cancer: JCOG 0509. J Clin Oncol 2014;32:1262-8. [PubMed]
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012-9. [PubMed]
- Oh I, Kim K, Kim Y, et al. Comparative Study Between Belotecan/Cisplatin and Etoposide/Cisplatin (COMBAT) in Patients with Previously Untreated, Extensive Stage Small Cell Lung Cancer. Sydney, Australia: IASCL - 15th World Conference on Lung Cancer, 2013. Available online: http://library.iaslc.org/virtual-library-search?product_id=5&author=&category=&date=&session_type=Poster+Session&session=p3&presentation=10-031&keyword=
- Jalal SI, Einhorn LH, Lo G, et al. Results from a randomized study of carboplatin and etoposide (CE) with or without palifosfamide (Pa) in extensive stage small cell lung cancer (ES-SCLC): The MATISSE study. J Clin Oncol 2015;33:abstr 7504.
- Ciuleanu T, Samarzjia M, Demidchik Y, et al. Randomized phase III study (SPEAR) of picoplatin plus best supportive care (BSC) or BSC alone in patients (pts) with SCLC refractory or progressive within 6 months after first-line platinum-based chemotherapy. J Clin Oncol 2010;28:15s.
- Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial. Ann Oncol 2015;26:908-14. [PubMed]
- Allen JW, Moon J, Redman M, et al. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol 2014;32:2463-70. [PubMed]
- Lee SM, Woll PJ, Rudd R, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst 2009;101:1049-57. [PubMed]
- Ready NE, Pang HH, Gu L, et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance). J Clin Oncol 2015;33:1660-5. [PubMed]
- Gitlitz BJ, Moon J, Glisson BS, et al. Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: a Southwest Oncology Group (SWOG 0435) phase II trial. J Thorac Oncol 2010;5:1835-40. [PubMed]
- Schneider BJ, Kalemkerian GP, Ramnath N, et al. Phase II trial of imatinib maintenance therapy after irinotecan and cisplatin in patients with c-Kit-positive, extensive-stage small-cell lung cancer. Clin Lung Cancer 2010;11:223-7. [PubMed]
- Pandya KJ, Dahlberg S, Hidalgo M, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: a trial of the Eastern Cooperative Oncology Group (E1500). J Thorac Oncol 2007;2:1036-41. [PubMed]
- Moore AM, Einhorn LH, Estes D, et al. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: a Hoosier Oncology Group phase II trial. Lung Cancer 2006;52:93-7. [PubMed]
- Miller AA, Pang H, Hodgson L, et al. A phase II study of dasatinib in patients with chemosensitive relapsed small cell lung cancer (Cancer and Leukemia Group B 30602). J Thorac Oncol 2010;5:380-4. [PubMed]
- Tarhini A, Kotsakis A, Gooding W, et al. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res 2010;16:5900-7. [PubMed]
- Belani CP, Dahlberg SE, Rudin CM, et al. Three-arm randomized phase II study of cisplatin and etoposide (CE) versus CE with either vismodegib (V) or cixutumumab (Cx) for patients with extensive stage-small cell lung cancer (ES-SCLC) (ECOG 1508). J Clin Oncol 2013;31:abstr 7508.
- Heymach JV, Johnson DH, Khuri FR, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann Oncol 2004;15:1187-93. [PubMed]
- Shepherd FA, Giaccone G, Seymour L, et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol 2002;20:4434-9. [PubMed]
- Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol 2008;26:870-6. [PubMed]
- Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 2012;18:3163-9. [PubMed]
- Lara PN Jr, Chansky K, Davies AM, et al. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327). J Thorac Oncol 2006;1:996-1001. [PubMed]
- Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol 2007;25:4278-84. [PubMed]
- Otterson GA, Hodgson L, Pang H, et al. Phase II study of the histone deacetylase inhibitor Romidepsin in relapsed small cell lung cancer (Cancer and Leukemia Group B 30304). J Thorac Oncol 2010;5:1644-8. [PubMed]
- Baggstrom MQ, Qi Y, Koczywas M, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol 2011;6:1757-60. [PubMed]
- Kotsakis A, Karavasilis V, Agelaki S, et al. Pazopanib as Second Line Treatment of Platinum Sensitive SCLC Patients: A Multicenter Phase II Trial of the Hellenic Oncology Research Group. Denver: WCLC: 16th World Conference on Lung Cancer, 2015. ID: 1683. Available online: Pazopanib+as+second+linehttp://library.iaslc.org/search?search_keyword=
- Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother 2014;15:2385-96. [PubMed]
- Freidlin B, Korn EL. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol 2014;11:81-90. [PubMed]
- Teicher BA. Perspective: Opportunities in recalcitrant, rare and neglected tumors. Oncol Rep 2013;30:1030-4. [PubMed]
- Kwei KA, Baker JB, Pelham RJ. Modulators of sensitivity and resistance to inhibition of PI3K identified in a pharmacogenomic screen of the NCI-60 human tumor cell line collection. PLoS One 2012;7:e46518. [PubMed]
- Sos ML, Dietlein F, Peifer M, et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc Natl Acad Sci U S A 2012;109:17034-9. [PubMed]
- Cardnell RJ, Feng Y, Diao L, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 2013;19:6322-8. [PubMed]
- Jahchan NS, Dudley JT, Mazur PK, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov 2013;3:1364-77. [PubMed]
- Haibe-Kains B, El-Hachem N, Birkbak NJ, et al. Inconsistency in large pharmacogenomic studies. Nature 2013;504:389-93. [PubMed]
- Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol 2015;16:395-405. [PubMed]
- Owonikoko TK, Zhang G, Deng X, et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med 2014;3:1579-94. [PubMed]
- Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66-70. [PubMed]
- Christensen CL, Kwiatkowski N, Abraham BJ, et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014;26:909-22. [PubMed]
- Dabir S, Wildey G, McColl K, et al. Identification of RICTOR amplification as a recurrent and potentially actionable alteration in small cell lung cancer patients. J Clin Oncol 2015;33:abstr 7576.
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136.
- Rudin CM, Pietanza M, Spigel DR, et al. A DLL3-Targeted ADC, Rovalpituzumab Tesirine, Demonstrates Substantial Activity in a Phase I Study in Relapsed and Refractory SCLC. Available online: https://cslide.ctimeetingtech.com/library/wclc/mylibrary/search/author/0/D.%20R.%20Spigel
- Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol 2013;8:587-98. [PubMed]
- Ruotsalainen TM, Halme M, Tamminen K, et al. Concomitant chemotherapy and IFN-alpha for small cell lung cancer: a randomized multicenter phase III study. J Interferon Cytokine Res 1999;19:253-9. [PubMed]
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- Ott PA, Elez-Fernandez ME, Hiret S, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 2015;33:abstr 7502.
- Antonia SJ, Bendell JC, Taylor MH, et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 2015;33:abstr 7503.


