Cancer stem cells in small cell lung cancer
Introduction
Lung cancer remains the most common cause of cancer-related death in the world. Small cell lung cancer (SCLC) occurs most frequently in smokers, accounting for 10–20% of all lung cancers and is one of the most malignant and aggressive lung tumors (1-3), killing around 20,000 people per year in the USA alone (4,5). SCLC patients usually present with metastasis to many organs, including the brain, at the time of diagnosis. The standard treatment is chemotherapy with cisplatin or carboplatin and etoposide and radiotherapy. However, despite the fact that patients initially have a good response the vast majority relapse, with a 1-year survival rate of 40%, and 5-year survival under 5% (6). Treated patients can be divided in two groups: those who progress after first line chemotherapy or within 60 days of the same are refractory cases; those who respond to first line therapy but relapse after 60 days are considered sensitive. Sensitive patients are more likely to respond to second-line chemotherapy than refractory patients (7). A variety of drugs are used in second-line chemotherapy including etoposide (8), irinotecan (9), gemcitabine (10), pemetrexed (11), paclitaxel (12), picoplatin (13), bendamustine (14) and topotecan or amrubicin (15). Although advances in molecular profiling and development of targeted therapies for non-small cell lung cancer (NSCLC) have progressed in recent years, in SCLC treatment advances remain non satisfactory (16): there have been no significant advances in the last 30 years with the only exception being surgical resection which only benefits a minority of carefully selected patients, but is not a standard treatment (17). Therefore, there is a clear need to find new therapeutic strategies to treat SCLC.
SCLC is a neuroendocrine cancer that secretes and responds to a wide variety of mitogenic peptide growth factors (18,19). It is composed of cells capable of differentiation into neuronal and endocrine lineages and with high proliferative capacity (20); SCLC has many genomic aberrations. For example, there are very frequent inactivating mutations in TP53 and Rb1 genes, but activating mutations in EGFR, KRAS, PIK3CA genes, c-Myc amplification, c-KIT overexpression and mutation/loss of PTEN are rare (21-24). In a study analyzing 51 SCLC samples, genetic alterations in PIK3CA pathway (36%) and PIK3CA mutations (6%) were also described (25). In another study in 60 SCLCs, PIK3CA was again identified as one of the prevalent aberrant genes (26). In a third study of 80 human SCLCs, including 40 SCLC cell lines, it was found that TP53 and Rb1 genes were frequently mutated. This study also detected SOX2 amplification/overexpression (27%) and RLF-MYCL1 gene fusions (9%) (27). Finally, in 99 SCLC samples analyzed it was found that in most samples TP53, and Rb1 genes had inactivating mutations. An addition, PTEN was mutated (10%) and there were inactivating mutations in EP300 and CREBBP, and the MYC and FGFR1 gene showed amplification (28).
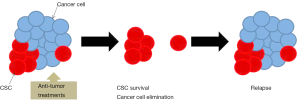
The cancer stem cell (CSC) model was proposed over 30 years ago (29) and is a very important field of study in cancer research. CSCs constitute a fraction of the total cancer cell population with frequency varying from 27% to 100% in highly tumorigenic cancers like haematopoietic and melanoma primary tumors, as well as in some cancer cell lines (30). However, for most of solid tumors CSCs account for less than 1% of the total cells (31). CSCs are characterized by capacity of self-renewal, asymmetric cell division, slow division kinetics, increased capacity of invasion, metastasis, tumor formation and proliferation, resistance to conventional chemotherapy and radiotherapy and can be identified by a variety of cell markers (32,33). Some characteristics of SCLC such as its aggressiveness, ability to differentiate into multiple lineages and develop of resistance to different treatments suggest that this tumor could be enriched in CSCs. Drug resistance in SCLC could be attributable to the existence of a resistant CSC subpopulation (Figure 1).
Evidence of CSC markers in SCLC
The ability to exclude Hoechst dye as defined by side population (SP) fraction was first described in normal haematopoietic cells (34), as well as in haematopoietic malignances and solid tumors (35). Less than 0.1% of total marrow cells are SP cells and these are enriched in drug-resistant haematopoietic stem cells (33). Several CSC characteristics are associated with SP fraction cells, such as the aforementioned ability to exclude Hoescht dye which is associated with high expression of drug transporters like ABC transporter family, including ABCB1 and ABCG2 that are able to exclude Hoescht dye from the cell (34). Another common characteristic is the association of drug transporter expression with drug resistance. Finally, SP fraction is enriched in cells capable of self-renewal and differentiation with reconstitution of the original cell (35) population similar to stem cells. These SP fractions with CSC features have been isolated in several different tumors.
As a SCLC model, Salcido et al. used the established SCLC cell lines NCI-H82, H146 and H526 and performed molecular characterization of SP cells with CSC features among these cells, showing that cell lines in this group had a low SP fraction (<1% of bulk cell population). This cell fraction had high proliferative capacity, efficient self-renewal and reduced cell surface expression of neuronal differentiation markers, CD56 and CD90, as compared with non-SP cells. They also formed more and faster growing tumors than non-SP cells. SP cells over-express many genes associated with CSCs and drug resistance, such as ABCG2, FGF1, IGF1, MYC, SOX1, SOX2 and WNTI, as well as genes involved in angiogenesis and the Notch and Hedgehog pathways (31). The Hedgehog signaling pathway is known to be active in SCLC tumors and its blockade reduces growth of SCLC tumor xenografts in mice (36). Also, Watkins et al. reported the importance of Hedgehog signaling in a subset of SCLC (37).
Wang et al. established a panel of lung cancer cell lines from primary tumors and characterized a small subpopulation strongly positive for CD44 (CD44high), with the main population being weakly positive or negative for CD44. Co-expression of CD90 (CD90+) further narrowed down the putative stem cell population. This CD44 and CD90 positive subpopulation showed mesenchymal morphology, increased expression of the mesenchymal markers vimentin and N-cadherin, increased mRNA levels of the embryonic stem cell-related genes Nanog and Oct4, and resistance to irradiation compared with other subpopulations. The CD44high CD90+ subpopulation is therefore a good candidate for a CSC marker (32).
Zhang et al. studied the SCLC cell line NCI-H446 and observed a high degree of stemness, tumorigenicity and plasticity. Stem cell markers detected were CD133, Sall4, Oct4, Nestin, neural cell adhesion molecule (NCAM), S100β, vimentin, CD44 and CD105. These cells form subcutaneous xenograft tumors and orthotopic lung xenograft tumors in BALB/C-nude mice and expressed stem cell markers and the cell nuclear antigen and proliferation marker Ki67 (38).
It has been commented that SOX2 has a role in maintaining the pluripotent stem cell phenotype (39). There are some clinically conflicting results regarding SOX2 expression, possibly either due to tumor-specific behavior of SOX2 or technical reasons. In one study, SOX2 protein expression was shown to be an independent marker for worse outcome in early stage lung adenocarcinoma (ADC) (40). In another, a relation between SOX2 expression and advanced disease, as well as worse overall survival (OS) in SCLC was found. However, it has been shown that SOX2 expression correlates with lower grade and with better outcome in SCLC (41); SOX2 protein expression has been related to more aggressive tumors (40,42-44). In addition, upregulation of SOX2 enhances tumor cell proliferation and SOX2 overexpression has been shown to be essential for lung CSC function (45,46).
Sarvi et al. characterized CD133 expression in H345 and H69 cell lines, in mouse models and human SCLCs. CD133 has been described as a CSC marker in other tumors and its expression correlated with chemoresistance to etoposide and increased tumorigenicity accompanied by increased expression of CD133 in human SCLC lung biopsy samples following chemotherapy. In addition, CD133 positive cells express increased neuro peptide receptors for gastrin-releasing peptide and arginine vasopressin (47). In another study, Eramo et al. showed that CD133 is also a useful marker in SCLC (48).
Roudi et al. studied the stem cell marker and cell adhesion molecule CD44 in different histological subtypes of lung cancer, analyzing 195 lung tumor samples, including 37 SCLC samples, by immunochemistry (IHC). Univariate analysis demonstrated that CD44 expression was higher in NSCLC compared to SCLC. In NSCLC, a higher level of CD44 expression was found in squamous cell carcinomas (SCC) compared to ADC. Higher CD44 expression correlated with higher grade tumors which in turn correspond to poor prognosis in SCC, and the lower level of CD44 expression was more often found in well differentiated ADCs. Also, high CD44 expression was associated with decreased levels of the proliferative marker Ki67 (49).
Roudi et al. also investigated CD133 and ALDH1 stem cell marker expression in lung cancer patients and found that ALDH1 and CD133 had higher expression in NSCLC compared to SCLC. High expression of ALDH1 and CD133 could be considered to be a CSC marker in some lung cancer subtypes such as SCC and ADC (50). Jiang et al. demonstrated that achaete-scute complex homolog 1 (ASCL1) regulates ALDH1 and CD133 and that CD133high-ALDH1high-ASCL1high subpopulation had CSC features in vitro and in vivo (51).
Wang et al. characterize a SP fraction in the H446 SCLC cell line and found 6.3% of SP cells by flow cytometry. They also found that SP cells were able to form tumor spheres better than non-SP cells. mRNA expression of the CSC markers ABCG2, CD133 and nucleostemin was analyzed and found to be 21.6, 7.1 and 1.02 higher than in non SP cells, respectively. SP cells have a greater ability to form tumors when compared with non SP cells and showed better proliferative ability and tougher viability when treated with drugs. Also, SP cells were able to differentiate in non-SP cells. The H446 cell line contains a CSC subpopulation, suggesting CD133 as a CSC marker in SCLC (52).
Miao et al. also used the H446 SCLC cell line as a model. However, they compared miRNA in stem-like cells and differentiated SCLC cells and studied expression of 1212 miRNAs in sphere-forming cells and parental cells by microRNA microarrays, in an enriched CSC subpopulation. They found 86 differentially expressed miRNAs (48 upregulated and 38 downregulated) and showed that downregulation of miR-27 enhances stem-like properties of SCLC cells and could be critical to maintaining stem cell function in SCLC (53).
Qiu et al. have also worked with the H446 cell line. After enrichment, the stem cell subpopulation showed an increase of stem cell markers urokinase plasminogen activator receptor (uPAR) and CD133 compared with parental cells. uPAR positive cells efficiently formed transplantable tumors, and could be differentiated into positive CD56, CK positive and uPAR negative cells. Therefore, uPAR and CD133 could function as CSC markers in SCLC (54). Gutova et al. reported that SCLC cells positive for uPAR were resistant to conventional chemotherapy and speculated that they contain a CSC subpopulation (55).
Kubo et al. also studied CD133 and CD87 like CSC markers in a panel of six SCLC cell lines, of which the SBC-7 cell line showed the highest expression levels of both markers. They isolated the CD133+/CD87−, CD133−/CD87+ subpopulations, and found that CD133−/CD87−. CD133+/CD87−, CD133−/CD87+ cells were more resistant to etoposide and paclitaxel and had greater repopulating ability than CD133−/CD87− cells. CD133+/CD87− cells contained more G0 quiescent cells than the CD133−/CD87− cells but CD133−/CD87− cells showed the highest tumorigenic potential. The researchers therefore concluded that CD133 and CD87 are inadequate CSC markers in SCLC (56).
Coe et al. described how disruption of the E2F/Rb pathway was deregulated in 96% of the SCLC samples investigated and was strongly associated with increased expression of EZH2, an oncogene and core member of the polycomb repressive complex (PRC2). EZH2 is epigenetically functionally active in SCLC, is pro-tumorigenic and associated with aberrant methylation profiles of PRC2 target genes, indicative of a stem-like hypermethylation profile in SCLC (57).
Morise et al. retrospectively studied expression of CSC makers such as Caveolin, Notch, CD44, CD166, SOX2, ALDH1 and Musashi 1 in patients who underwent surgical resection of SCLC (n=60) and large cell neuroendocrine carcinoma (LCNEC) (n=45). They found a difference between SCLC and LCNEC, with regard to both SOX2 (55% vs. 27%, P=0.003) and CD166 (27% vs. 47%, P=0.034). ALDH1 expression was similar in SCLC and LCNEC (67% vs. 73%, P=0.46) and ALDH positive patients had significantly worse recurrence-free survival (RFS) and OS rates compared with ALDH negative patients (5-year RFS: 39% vs. 67%, P=0.009; 5-year OS: 50% vs. 79%, P=0.021). A multivariate analysis revealed that positive ALDH expression was an independent unfavorable prognostic factor with regard to both RFS and OS (58).
PODXL-1 and Bmi1 are markers in hematopoietic stem cells. Koch et al. studied their expression by IHC in 64 SCLC samples and demonstrated that 56 were positive for PODXL-1 and Bmi1. They hypothesized that both could be CSC markers for SCLC (59).
Immunotherapy and other anti-CSC therapies in SCLC
The objective of immunotherapy is to stimulate the immune system and detect and destroy cancer cells. It can be used alone or in combination with chemotherapy and often produces a durable response in small subpopulations of patients. Successful treatment with immunotherapy for NSCLC indicates that similar results are also possible in SCLC and this is an active ongoing area of research. In contrast to what was previously believed, a body of evidence now exists to suggest that lung cancer is an immunogenic disease (60). In extended-SCLC patients show significant clinical deterioration with rapid progressive disease and for this reason there is no time to get an appropriate immune response and then is necessary to get an adequate schedule of immunotherapy in relation to chemotherapy (61).
Several first-line therapies have been tested in SCLC, such as inhibitors of angiogenesis and growth factor receptors, promoters of apoptosis and p53 cancer vaccines. However, most trials have failed to show improved PFS and OS (15). New cancer vaccines, adoptive immunotherapy, cytokines and checkpoint inhibitors have also now been tested in clinical trials in SCLC.
A phase II study tested NTX-010, a Seneca Valley virus with specific tropism for neuroendocrine markers in SCLC. SCLC patients who did not progress following four cycles of induction platinum therapy were randomized to NTX-010 or placebo. However, median PFS was identical in both arms at 1.7 months (62).
Immune checkpoints are inhibitory pathways used by tumors to escape to immune system control (63). Inhibition of the immune checkpoints releases the brakes on the immune system, resulting in antigen-specific T-cell responses (64). In lung cancer, targeting CTLA4 (an immunomodulatory molecule expressed in T cells), PD-1 and its ligand PD-L1 has shown promising and durable responses (65). PD-L1 is not expressed in SCLC tumor cells but is in tumor infiltrating macrophages (66). One strategy is to target CSCs with monoclonal antibodies targeting antigens differentially overexpressed in tumor cells. Ipilimumab is a human monoclonal antibody that blocks binding of CTL-4 to its ligand. A phase III study compares etoposide versus etoposide plus ipilimumab to enhance T cell responses and prolong OS (61). A phase II clinical assay in SCLC patients (n=130) used the CTLA-4 antagonist ipilimumab combined with carboplatin and paclitaxel and showed improved immune-related PFS (HR 0.64, P=0.03) and median OS (12.9 vs. 9.9 months) compared to control patients (67). In A phase III study (68) is now ongoing as is one testing the combination of ipilimumab plus nivolumab, a human monoclonal antibody that binds to PD-1 (69).
PD-L1 expression has been correlated with longer survival in SCLC patients (70). The anti-PD-L1 antibody MEDI4736 has been evaluated in a phase I/II study (71) and a phase I study of MEDI4736 in combination with the anti-CTLA-4 antibody tremelimumab is also ongoing in patients with advanced solid tumors (NCT02261220).
Specific gangliosides are highly expressed in SCLC and are potential targets for immunologic therapies. A phase I/II study with the monoclonal antibody FucGM1 is ongoing (72).
GD3 is a glycosphingolipid antigen highly expressed in SCLC (73) and BEC2 is a monoclonal antibody that mimics GD3. In a clinical trial, BEC2 showed promising results (74), but another trial, where 515 patients were included, showed no significant statistical difference in median OS (75).
Cellular immunotherapy (CIT) has been showed to be effective for several tumors. Ding et al. demonstrated that CIT as maintenance therapy prolongs the survival of SCLC patients (76).
Epigenetic changes could be regulated by mutations including chromatin modifiers and epigenetic readers. Methylation regulates key SCLC genes like BCL2 overexpression and RB1 silencing (77). In SCLC, preclinical activity of vorinostat and belinostat histone deacetylase inhibitors in combination with cisplatin and etoposide (standard chemotherapy for SCLC) or topotecan (approved as second-line therapy) is driving new clinical trials with these drugs (77). LSD1 is a histone modifier that maintains the pluripotency of embryonic stem cells through demethylation of histone H3 lysine 4 (H3K4) and subsequently repression of genes controlling cell differentiation (78). LSD1 is overexpressed in many tumors including SCLC (79). Due to the central role of LSD1 in stem cell maintenance and cancer progression, there has been a drive to identify LSD1 inhibitors. Mohammad et al. used the GSK2879552 LSD1 inhibitor in a panel of 165 cancer cell lines representing multiple cancer cell types and found that a subset of SCLC cell lines were sensitive. GSK2879552 was cytostatic, rather than cytotoxic, resulting in delayed onset of growth inhibition. In addition, in SCLC xenograft models, tumors did not significantly regress, but growth was highly delayed when animals were treated with GSK2879552. This drug causes a change in the expression of genes involved in neuroendocrine differentiation, a hallmark of SCLC. Researchers also found LSD1 and H3K4 methylation enrichment surrounding transcriptional start sites of genes involved in regulation of cell state. In summary, these results demonstrated that LSD1 plays a role in maintaining SCLC stemness, and that inhibition of this molecule in preclinical models reduced cell proliferation and CSCs while promoting cell differentiation and reducing tumor growth. Since only a subgroup of SCLC models were sensitive to LSD1 inhibition, the investigators tried to find markers to select these sensitive subgroups. They failed to identify RNA markers but found 45 methylation probes with differences between sensitive and resistant models which serve to separate SCLC tumor models into two groups. They used this methylation signature to predict the response of three patient-derived xenografts (PDX) to treatment with GSK2877552. A phase I study of this drug in patients with relapsed/refractory SCLC is now ongoing (NCT02034123) (77).
As previously commented, Sarvi et al. found that CD133 positive cells express increased neuropeptide receptors (47). In a phase I clinical trial, a novel broad spectrum neuropeptide antagonist [related substance P analogue (SP-G)] was tested but shown to have a short half-life and poor bioavailability (80,81). Sarvi et al. synthetized a panel of modified analogues based on the structure of SP-G and tested them in vitro and in vivo. One of the analogues, Peptide-1, showed increased inhibition of cell growth, induced more apoptosis in the SCLC cell lines H345 and H69 when compared with SP-G and was is four times more stable than SP-G. When Peptide-1 was tested in the H345 xenograft model it produced a significant reduction in tumor volume for the duration of the study and was as least as efficacious as the chemotherapy drug etoposide. Tumors treated with Peptide-1 showed very few CD133 positive cells compared with tumors treated with etoposide. For this reason Sarvi et al. proposed Peptide-1 as an anticancer agent with greater efficacy in resistant and CD133 positive SCLC tumors (47).
VS-5584 is a selective inhibitor of mTORC1/2 and class I PI3K kinases. Kolev et al. described how VS-5584 is 30-fold more potent in inhibiting proliferation and survival of CSC compared with non-CSC in solid tumor cell populations. They tested the drug in a NCI-H841 SCLC xenograft model and found that VS-5584 caused significant growth inhibition and decrease of SP cells, indicating a reduced proportion of CSC in NCI-H841 tumors. In addition, cells dissociated from NCI-H841 tumors in mice treated with VS-5584 showed a 67-fold reduction in tumor-initiating frequency when these cells were injected in limited dilutions into immunodeficient mice, indicating a marked depletion of CSC. Following these experiments, Kolev et al. studied the effects of VS-5584 after treatment with cisplatin or etoposide (82). Prior experiments showed that neither cisplatin nor etoposide were effective in depleting CSC of SCLC cell lines; in fact these two drugs enriched the CSC population. In a NCI-H69 SCLC xenograft model, weekly intraperitoneal dosing of 5 mg/kg of cisplatin for 2 weeks induced initial tumor regression but tumors re-grew quickly after treatment cessation (83). When VS-5584 was given orally at 15 mg/kg in this xenograft model significant inhibition of NCI-H69 tumor growth was observed. When VS-5584 was tested following cessation of cisplatin treatment, a delay in regrowth of NCI-H69 tumors was observed. VS-5584 was tested in a PDX model, established from an SCLC lymph node metastasis. When VS-5584 was administered following cessation of cisplatin tumor regrowth was delayed (84).
The Notch signaling pathway has also been shown to regulate normal stem cells and neoplastic transformation when deregulated (85). In the phase Ib/II “PINACLE” trial, an anti-Notch 2/3 was tested in combination with etoposide and cisplatin in first-line extensive-stage SCLC patients (86).
Conclusions
SCLC remains one the most aggressive tumors, with poor prognosis. Nowadays, standard chemotherapy (cisplatin/etoposide in first-line, topotecan in second-line) are the standard treatments, however, new therapies are urgently required. In order to validate new drugs in SCLC, it is first necessary to elucidate the biological mechanisms that cause cancer promotion and progression. CSC theory is central to cancer cell biology and cancer therapy and is well supported in lung cancer since CSCs are associated with aggressive cancer behavior, metastatic progression, resistance to therapy and relapse. The ambiguity in the nature of heterogeneity among CSCs depends on the SCLC subtype tumor population studied. This lack of specificity in identifying CSC markers for the diverse CSCs pool may represent a major problem to translate the CSC model into clinical strategies. The discovery of specific CSC markers is crucial, and it is also essential to clarify the function of these molecules, as well as the signaling pathways and gene transcriptions that control CSC activity in order to design adequate drugs that attack CSCs. Several potential targets have been identified in SCLC, and several compounds are currently under investigation in vitro, in vivo and in clinical trials. The ability to exclude Hoechst dye defined as SP fraction is a criteria to describe CSCs since this subpopulation possesses some characteristic CSC features. Several possible CSC markers in SCLC have been described, such as CD44, CD90, CD133, CD87, OCT4, SOX2, ALDH1 and uPAR. With regard to the different treatments used to attack CSC subpopulations in SCLC, immunotherapy has now a promising role in NSCLC and is under investigation in SCLC. Ipilimumab, a CTLA-4 antagonist, combined with chemotherapy, has showed improved immune-related PFS and improved OS. Ipilimumab can also be combined with nivolumab, a PD-1 antagonist. In conclusion, new CSC-targeting compounds may be a promising strategy to prevent cancer recurrence and metastasis, however, more questions remain unanswered. It is necessary to discover adequate CSC markers to identify and stratify patient subgroups and then to accurately target these CSC subpopulations with therapies. In addition, it is important to detect new markers to predict better outcomes with the new therapeutic agents tested.
Acknowledgements
We are grateful for the diligent revision of our manuscript by Kate Williams.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003;41:245-58. [PubMed]
- Ferlay J, Randi G, Bosetti C, et al. Declining mortality from bladder cancer in Europe. BJU Int 2008;101:11-9. [PubMed]
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92. [PubMed]
- Rodriguez E, Lilenbaum RC. Small cell lung cancer: past, present, and future. Curr Oncol Rep 2010;12:327-34. [PubMed]
- Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J 2010;35:202-15. [PubMed]
- Haberland J, Bertz J, Wolf U, et al. German cancer statistics 2004. BMC Cancer 2010;10:52. [PubMed]
- von Pawel J, Ardizzoni A, Thatcher N. The relationship between treatment-free interval (TFI) and outcomes to therapy in patients with relapsed small cell lung cancer (SCLC): a review of 631 patients treated with iv topotecan in 6 studies. Lung Cancer 2003;41:S235.
- Evans WK, Feld R, Osoba D, et al. VP-16 alone and in combination with cisplatin in previously treated patients with small cell lung cancer. Cancer 1984;53:1461-6. [PubMed]
- Masuda N, Matsui K, Negoro S, et al. Combination of irinotecan and etoposide for treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1998;16:3329-34. [PubMed]
- Hoang T, Kim K, Jaslowski A, et al. Phase II study of second-line gemcitabine in sensitive or refractory small cell lung cancer. Lung Cancer 2003;42:97-102. [PubMed]
- Jalal S, Ansari R, Govindan R, et al. Pemetrexed in second line and beyond small cell lung cancer: a Hoosier Oncology Group phase II study. J Thorac Oncol 2009;4:93-6. [PubMed]
- Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347-51. [PubMed]
- Eckardt JR, Bentsion DL, Lipatov ON, et al. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J Clin Oncol 2009;27:2046-51. [PubMed]
- Schmittel A, Knödler M, Hortig P, et al. Phase II trial of second-line bendamustine chemotherapy in relapsed small cell lung cancer patients. Lung Cancer 2007;55:109-13. [PubMed]
- Asai N, Ohkuni Y, Kaneko N, et al. Relapsed small cell lung cancer: treatment options and latest developments. Ther Adv Med Oncol 2014;6:69-82. [PubMed]
- Lovly CM, Carbone DP. Lung cancer in 2010: One size does not fit all. Nat Rev Clin Oncol 2011;8:68-70. [PubMed]
- Ploenes T, Osei-Agyemang T, Krohn A, et al. Surgical treatment of early stage small cell lung cancer. Asian Cardiovasc Thorac Ann 2012;20:694-8. [PubMed]
- Sethi T, Langdon S, Smyth J, et al. Growth of small cell lung cancer cells: stimulation by multiple neuropeptides and inhibition by broad spectrum antagonists in vitro and in vivo. Cancer Res 1992;52:2737s-2742s. [PubMed]
- Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: effects of bradykinin, vasopressin, cholecystokinin, galanin, and neurotensin. Cancer Res 1991;51:3621-3. [PubMed]
- Sone S, Nakayama T, Honda T, et al. CT findings of early-stage small cell lung cancer in a low-dose CT screening programme. Lung Cancer 2007;56:207-15. [PubMed]
- Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol 2001;28:3-13. [PubMed]
- Shibata T, Kokubu A, Tsuta K, et al. Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett 2009;283:203-11. [PubMed]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 1998;17:475-9. [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [PubMed]
- Umemura S, Goto K, Mimaki S, et al. Comprehensive genomic analysis of small cell lung cancer in Asian patients. J Clin Oncol 2013;31:abstr 7512.
- Wakuda K, Kenmotsu H, Serizawa M, et al. Molecular profiling of small cell lung cancer in a Japanese cohort. Lung Cancer 2014;84:139-44. [PubMed]
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [PubMed]
- Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science 1977;197:461-3. [PubMed]
- Kelly PN, Dakic A, Adams JM, et al. Tumor growth need not be driven by rare cancer stem cells. Science 2007;317:337. [PubMed]
- Salcido CD, Larochelle A, Taylor BJ, et al. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer 2010;102:1636-44. [PubMed]
- Wang P, Gao Q, Suo Z, et al. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS One 2013;8:e57020. [PubMed]
- Sales KM, Winslet MC, Seifalian AM. Stem cells and cancer: an overview. Stem Cell Rev 2007;3:249-55. [PubMed]
- Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797-806. [PubMed]
- Hadnagy A, Gaboury L, Beaulieu R, et al. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res 2006;312:3701-10. [PubMed]
- Vestergaard J, Pedersen MW, Pedersen N, et al. Hedgehog signaling in small-cell lung cancer: frequent in vivo but a rare event in vitro. Lung Cancer 2006;52:281-90. [PubMed]
- Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003;422:313-7. [PubMed]
- Zhang Z, Zhou Y, Qian H, et al. Stemness and inducing differentiation of small cell lung cancer NCI-H446 cells. Cell Death Dis 2013;4:e633. [PubMed]
- Jung YW, Hysolli E, Kim KY, et al. Human induced pluripotent stem cells and neurodegenerative disease: prospects for novel therapies. Curr Opin Neurol 2012;25:125-30. [PubMed]
- Sholl LM, Barletta JA, Yeap BY, et al. Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am J Surg Pathol 2010;34:1193-8. [PubMed]
- Wilbertz T, Wagner P, Petersen K, et al. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol 2011;24:944-53. [PubMed]
- Wang Q, He W, Lu C, et al. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res 2009;29:1233-41. [PubMed]
- Saigusa S, Tanaka K, Toiyama Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol 2009;16:3488-98. [PubMed]
- Du L, Yang Y, Xiao X, et al. Sox2 nuclear expression is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral Oncol 2011;47:709-13. [PubMed]
- Xiang R, Liao D, Cheng T, et al. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer 2011;104:1410-7. [PubMed]
- Nakatsugawa M, Takahashi A, Hirohashi Y, et al. SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest 2011;91:1796-804. [PubMed]
- Sarvi S, Mackinnon AC, Avlonitis N, et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res 2014;74:1554-65. [PubMed]
- Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008;15:504-14. [PubMed]
- Roudi R, Madjd Z, Korourian A, et al. Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer Biomark 2014;14:457-67. [PubMed]
- Roudi R, Korourian A, Shariftabrizi A, et al. Differential Expression of Cancer Stem Cell Markers ALDH1 and CD133 in Various Lung Cancer Subtypes. Cancer Invest 2015;33:294-302. [PubMed]
- Jiang T, Collins BJ, Jin N, et al. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res 2009;69:845-54. [PubMed]
- Wang B, Yang H, Huang YZ, et al. Biologic characteristics of the side population of human small cell lung cancer cell line H446. Chin J Cancer 2010;29:254-60. [PubMed]
- Miao Y, Li J, Qiu X, et al. miR-27a regulates the self renewal of the H446 small cell lung cancer cell line in vitro. Oncol Rep 2013;29:161-8. [PubMed]
- Qiu X, Wang Z, Li Y, et al. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett 2012;323:161-70. [PubMed]
- Gutova M, Najbauer J, Gevorgyan A, et al. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS One 2007;2:e243. [PubMed]
- Kubo T, Takigawa N, Osawa M, et al. Subpopulation of small-cell lung cancer cells expressing CD133 and CD87 show resistance to chemotherapy. Cancer Sci 2013;104:78-84. [PubMed]
- Coe BP, Thu KL, Aviel-Ronen S, et al. Genomic deregulation of the E2F/Rb pathway leads to activation of the oncogene EZH2 in small cell lung cancer. PLoS One 2013;8:e71670. [PubMed]
- Morise M, Hishida T, Takahashi A, et al. Clinicopathological significance of cancer stem-like cell markers in high-grade neuroendocrine carcinoma of the lung. J Cancer Res Clin Oncol 2015;141:2121-30. [PubMed]
- Koch LK, Zhou H, Ellinger J, et al. Stem cell marker expression in small cell lung carcinoma and developing lung tissue. Hum Pathol 2008;39:1597-605. [PubMed]
- Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220-7. [PubMed]
- Massarelli E, Papadimitrakopoulou V, Welsh J, et al. Immunotherapy in lung cancer. Transl Lung Cancer Res 2014;3:53-63. [PubMed]
- Molina JR, Mandrekar SJ, Dy GK, et al. A randomized double-blind phase II study of the Seneca Valley virus (NTX-010) versus placebo for patients with extensive stage SCLC (ES-SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: Alliance (NCCTG) N0923 study. J Clin Oncol 2013;31:abstr 7509.
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90:297-339. [PubMed]
- Ascierto PA, Kalos M, Schaer DA, et al. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res 2013;19:1009-20. [PubMed]
- Karachaliou N, Cao MG, Teixidó C, et al. Understanding the function and dysfunction of the immune system in lung cancer: the role of immune checkpoints. Cancer Biol Med 2015;12:79-86. [PubMed]
- Schultheis AM, Scheel AH, Ozretić L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015;51:421-6. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- von Pawel J, Kim SW, Spigel DR, et al. CA184-156: Randomized, multicenter, double-blind, phase III trial comparing the efficacy of ipilimumab (Ipi) plus etoposide/platinum (EP) versus placebo plus EP in patients (Pts) with newly diagnosed extensive-stage disease small cell lung cancer (ED-SCLC). J Clin Oncol 2013;31:abstr TPS7608.
- Pillai RN, Owonikoko TK. Small cell lung cancer: therapies and targets. Semin Oncol 2014;41:133-42. [PubMed]
- Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426-30. [PubMed]
- Zielinski CC. A phase I study of MEDI4736, NNT-PD-L1 antibody in patients with advanced solid tumors. Transl Lung Cancer Res 2014;3:406-7. [PubMed]
- Krug LM, Ragupathi G, Hood C, et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res 2004;10:6094-100. [PubMed]
- Fuentes R, Allman R, Mason MD. Ganglioside expression in lung cancer cell lines. Lung Cancer 1997;18:21-33. [PubMed]
- Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guérin. Clin Cancer Res 1999;5:1319-23. [PubMed]
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- Ding X, Cao H, Chen X, et al. Cellular immunotherapy as maintenance therapy prolongs the survival of the patients with small cell lung cancer. J Transl Med 2015;13:158. [PubMed]
- Stewart CA, Byers LA. Altering the Course of Small Cell Lung Cancer: Targeting Cancer Stem Cells via LSD1 Inhibition. Cancer Cell 2015;28:4-6. [PubMed]
- Adamo A, Sesé B, Boue S, et al. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol 2011;13:652-9. [PubMed]
- Lv T, Yuan D, Miao X, et al. Over-expression of LSD1 promotes proliferation, migration and invasion in non-small cell lung cancer. PLoS One 2012;7:e35065. [PubMed]
- Jones DA, Cummings J, Langdon SP, et al. Metabolism of the anticancer peptide H-Arg-D-Trp-NmePhe-D-Trp-Leu-Met-NH2. Peptides 1995;16:777-83. [PubMed]
- Jones DA, Cummings J, Langdon SP, et al. Characterization of the deamidase enzyme responsible for the metabolism of the anticancer peptide: H-Arg-D-Trp-NmePhe-D-Trp-Leu-Met-NH2. Biochem Pharmacol 1995;50:585-90. [PubMed]
- Kolev VN, Wright QG, Vidal CM, et al. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res 2015;75:446-55. [PubMed]
- Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A 2000;97:14720-5. [PubMed]
- García Campelo MR, Alonso Curbera G, Aparicio Gallego G, et al. Stem cell and lung cancer development: blaming the Wnt, Hh and Notch signalling pathway. Clin Transl Oncol 2011;13:77-83. [PubMed]
- Espinoza I, Pochampally R, Xing F, et al. Notch signaling:targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther 2013;6:1249-59. [PubMed]
- Koren A, Motaln H, Cufer T. Lung cancer stem cells: a biological and clinical perspective. Cell Oncol (Dordr) 2013;36:265-75. [PubMed]


