Inhibition of insulin-like growth factor receptor: end of a targeted therapy?
Introduction
Lung cancer remains the most lethal form of cancer worldwide, with 1.37 million deaths in 2008 (1). Due to lack of effective screening programs until recently, most patients present with advanced disease, where the mainstay of therapy is chemotherapy. Frontline treatment for patients with lung cancer consists of platinum doublet therapy, based on seminal publications that established improvement in survival over best supportive care (2). Recent advances in the genetic characterization of lung cancers have resulted in use of targeted agents for specific subsets of NSCLC. However, the majority of patients still receive systemic chemotherapy since targetable molecular abnormality is detected only in approximately 20%. There is a great need to find new targets to improve the efficacy of treatment for lung cancer patients.
The insulin-like growth factor (IGF) pathway has been extensively studied as an important signaling pathway in cancer. IGF-1 and its receptor, the insulin growth factor 1 receptor (IGF-1R), have been implicated in carcinogenesis and to cancer risk in the population. IGF-1, produced in the liver, mediates the effects of growth hormone. IGF-1R is a member of the insulin receptor subclass of tyrosine kinase membrane receptors and shares structural homology with the insulin receptor (3). IGF-1R consists of a tetramer of two alpha subunits that bind IGF-1, and insulin less avidly, and two intracellular beta subunits, which have tyrosine kinase domains with the ATP binding site. Once IGF-1R binds to IGF-1, this causes a conformational change in the receptor, leading to activation of the kinase domain. IGF-1R can then signal through adapter proteins insulin receptor substrate (IRS) 1 and 2 to activate downstream targets including the PI3K/AKT/mTOR and Ras/Raf/MAPK pathways, leading to cell cycle progression, cell proliferation, and cell survival (Figure 1). Serum IGF-binding proteins (IGFBPs) bind 90% of circulating IGF-1 and prevent receptor binding and prolong half-life of IGF-1. IGF signaling has been implicated in regulation of angiogenesis, as this pathway can activate HIF-1α expression and VEGF secretion in lung cancer cell lines (3). There also appears to be crosstalk between IGF pathway and EGFR signaling; inhibition of the EGFR pathway is less effective in the presence of IGF-1R overexpression, suggesting a potential mechanism behind development of resistance to EGFR inhibitors.
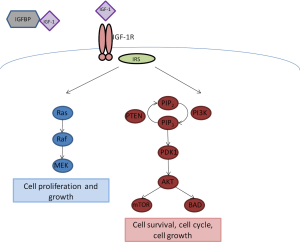 Figure 1. IGF-1R Signaling Pathway. IGF-1 is regulated by binding to insulin growth factor binding protein (IGFBP). Free IGF-1 can bind to IGF-1R, and activate downstream signaling through the insulin receptor substrate (IRS) to promote cell growth and proliferation (Ras/Raf/MEK pathway) and cell survival and cell cycle progression (PI3K/AKT/mTOR pathway)
Figure 1. IGF-1R Signaling Pathway. IGF-1 is regulated by binding to insulin growth factor binding protein (IGFBP). Free IGF-1 can bind to IGF-1R, and activate downstream signaling through the insulin receptor substrate (IRS) to promote cell growth and proliferation (Ras/Raf/MEK pathway) and cell survival and cell cycle progression (PI3K/AKT/mTOR pathway)
The IGF signaling pathway has been implicated in carcinogenesis and cancer risk in many studies. In a landmark paper, lack of IGF-1R expression in mouse fibroblasts resulted in loss of oncogenic transformation by simian virus 40 (SV40) large T antigen expression (4). Wild-type fibroblasts were transformed by SV40 large T antigen expression and able to form foci in both culture and soft agar, demonstrating hallmarks of cancer cells with loss of contact inhibition and anchorage-independent growth. These findings were further verified by the loss of transformation seen in fibroblasts treated with antisense RNA against IGF-1R. This was the first demonstration of the importance of IGF-1R in carcinogenesis.
IGF-1 was also shown to be important for tumor growth in a breast cancer xenograft model in mice; mice that were homozygous for lit mutation, resulting in loss of IGF-1/growth hormone axis, had significantly smaller tumors than in control mice (5). With regards to lung cancer, IGF overexpression in transgenic mice led to the development of adenomatous hyperplasia (6). IGF-1R is overexpressed in cancer cell lines and in human cancers (3,7). IGF-1 and IGF-1R overexpression has been studied in multiple epidemiological studies and linked to increased risk of lung, ovarian, pancreatic, breast, and colorectal cancers (7).
Clinical trials with IGF-1R inhibitors in lung cancer
Based on the sound pre-clinical rationale supporting IGF-1R as a therapeutic target, agents that inhibit IGF-1R were evaluated in clinical trials (Table 1). The majority of the studies conducted to date have evaluated monoclonal antibodies that bind to, and inhibit the IGF-1R. Presently, small molecule tyrosine kinase inhibitors of IGF-1R are also under development.
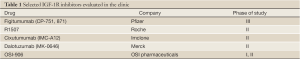
Full table
Figitumumab
The first specific IGF-1R antagonist which was developed in the clinic is figitumumab (Table 2), previously known as CP-751,871, a fully humanized monoclonal antibody against IGF-1R (Pfizer, New London, CT). Phase II data were published in 2009, which showed very promising activity in combination with platinum doublet chemotherapy (8). Patients with chemotherapy-naïve advanced and metastatic non-small cell lung cancer (NSCLC) were randomized 2:1 to carboplatin/paclitaxel/figitumumab (PCF) or carboplatin/paclitaxel (PC). Carboplatin was dosed to achieve an area under the plasma concentration-time curve (AUC) of 6 and paclitaxel at 200 mg/m2 every 3 weeks; figitumumab was given to the first cohort of patients at 10 mg/kg IV and second cohort at 20 mg/kg IV, every 3 weeks. Patients in the control arm could receive figitumumab with or without PC per investigator’s discretion at progression (n=20 patients). Patients in the treatment arm could continue on single agent figitumumab after discontinuation of chemotherapy (n=47 patients). The study demonstrated an objective response rate (ORR) of 54% with PCF compared to 42% in PC arm. The most remarkable findings were from a subgroup analysis: patients with squamous cell carcinoma were found to have an ORR of 78% and 12-week progression-free survival (PFS) rate of 89% with figitumumab 20 mg/kg. An additional 30 patients were enrolled to single arm expansion cohort to confirm these results. There was no difference in PFS between the PCF and PC groups, but for patients in the control arm that received figitumumab at progression, this translated into improved PFS with HR 0.56 (P=0.0153, 95% CI, 0.28 to 0.87). The combination therapy was associated with a higher incidence of grade 3 and 4 hyperglycemia seen in PCF arm (15%) compared to PC arm (8%). There were eight deaths during study treatment, with 5 reported in the PCF arm compared to 3 deaths in PC. Based on these phase II data, there was great excitement in proceeding with a phase III, particularly in patients with squamous cell histology, for whom there have been few successful therapies.
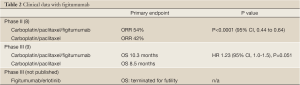
Full table
The results of the phase III study were presented at the 2010 American Society for Clinical Oncology (ASCO) meeting (9). The planned enrollment was 820 patients with non-adenocarcinoma NSCLC to be randomized to PCF with 20 mg/kg or PC in the first line setting. The primary endpoint was overall survival (OS). The study was terminated after 681 patients (86% squamous and 88% stage IV) were enrolled based on a planned interim analysis that demonstrated a hazard ratio that crossed futility boundary favoring PC. The OS in PCF was 10.3 months compared to 8.5 months in PC arm (HR 1.23, 95% CI, 1.0-1.5, P=0.051). In patients with serum IGF-1 level greater than or equal to 1 ng/mL, OS was 10.2 months in PCF group compared to 7 months in PC group (HR 0.97, 95% CI, 0.6-1.7). The OS hazard ratio favored PC in patients with low baseline IGF-1 (HR 1.6 PCF/PC ratio for patients with IGF1 <120 ng/mL, P=0.006) and PCF in patients with high baseline IGF1 levels (over 145 ng/mL, HR=0.62, P=0.13). The disappointing results of the phase III study were likely a consequence of the lack of predictive biomarkers for patient selection, particularly in light of the absence of survival benefit in the preceding phase II study.
Based on the correlation between efficacy of figitumumab and the serum IGF-1 levels in the Jassem study, biomarker analysis was done in the serum specimens collected from patients that participated in the phase II study described earlier (10). The study team evaluated IGF-1, IGF-2, IGFBP1-3, insulin, and cotinine in plasma samples obtained from patients. Of all these, high pre-treatment levels of free IGF-1 defined as at least 0.54 ng/mL were found to correlate with improved PFS (P=0.007) in patients who received 20 mg/kg dose of figitumumab. Conversely, low levels of free IGF-1 was associated with a median PFS of <3 months (P=0.026). For patients treated with chemotherapy alone, IGF-1 levels were not predictive of PFS improvement, suggesting that the marker is specific for therapy with figitumumab. The authors found that a threshold of free IGF-1 level above 0.7 ng/mL predicted different median PFS by treatment group: 2.63 months with chemotherapy alone, 3.97 with PCF using 10 mg/kg figitumumab dose, and 6.53 months in PCI group who received 20 mg/kg figitumumab. In patients with free IGF-1 levels below 0.7 ng/mL, there was no difference in median PFS between the three treatment groups. There was a correlation between high free IGF-1 levels and expression of vimentin, a mesenchymal marker that is linked with epithelial to mesenchymal transition (EMT). The results from these two studies suggest that baseline serum IGF-1 level could help select patients that might benefit from figitumumab combination. These results may be useful to the clinical development of other IGF-1R inhibitors.
Figitumumab was also studied in combination with erlotinib in the phase III ADVIGO 1018 study. This trial enrolled patients with advanced NSCLC with non-adenocarcinoma histology in the second or third line setting. The study was halted due to an interim analysis which demonstrated the futility of this combination in March 2010 (11). As the data have never been presented, it is unclear what factors may have impacted the disappointing results.
Clinical data with figitumumab
R1507
R1507 is a humanized recombinant monoclonal antibody against IGF-1R developed by Roche (Basel, Switzerland). Based on preclinical observations that IGF-1R signaling interacts with EGFR signaling and may mediate resistance to EGFR inhibitors, R1507 was studied in a phase II trial in combination with erlotinib in patients with advanced NSCLC (12). Patients with metastatic NSCLC that had progressed on one or two prior chemotherapy regimens were randomized to erlotinib 150 mg orally daily in combination with either R1507 9 mg/kg IV weekly, 16 mg/kg IV every 3 weeks or placebo. The primary endpoint was PFS rate at 12 weeks. Patient tumor samples were tested for activating EGFR mutations and KRAS mutations. In this patient population of predominantly male patients (65-68%) with a minority of never smokers (9-16%), there was no difference in 12-week PFS rate between the treatment groups: 41% with erlotinib alone, 42% erlotinib and weekly R1507, and 45% erlotinib and every 3 weekly R1507. The OS was 8.1 months in erlotinib alone, 8.1 months erlotinib and weekly R1507, and 12.1 months with erlotinib and 3 week R1507, which was not statistically significant: the hazard ratio was 0.84 with weekly R1507 (0.58 to 1.21, 90% CI, P=0.43) and 0.72 with three weekly R1507 (0.53 to 0.99, 90% CI, P=0.09). However, in the 27% of patients with mutated KRAS, the 12-week PFS rate was improved to 36% in patients who received R1507 compared to 0 in patients treated with placebo (P=0.039). It is interesting that the KRAS mutated patients seem to benefit from the addition of IGF-1 inhibitor R1507 to EGFR inhibition, as these patients are typically resistant to EGFR inhibition. The results suggest that patients with KRAS mutations may benefit from combined inhibition of the IGF-1R and EGFR pathways.
In order to determine other predictive biomarkers for treatment with R1507 and erlotinib, archived tumor tissue and plasma were assessed for free and total IGF-1, IGFBP-3, IGF-1R, pAKT, PTEN, EGFR, and KRAS and correlated with the primary endpoint of the study, 12-week PFS rate (13). Free IGF-1 level was significantly correlated with improved 12-week PFS rate in the patients treated in the 16 mg/kg dose of R1507: 46% patients with elevated free IGF-1 level treated with R1507 achieved 12 week PFS compared to 18% patients in placebo arm, HR=3.94 (95% CI, 1.2-13.6). None of the other biomarkers had a significant impact on treatment response with R1507. These results further substantiate the observations that high levels of serum IGF-1 might be a useful selection parameter for treatment with IGF-1R inhibitors.
Cixutumumab
Cixutumumab (IMC-A12) is a fully humanized monoclonal IgG1 antibody against IGF-1R developed by Imclone (Bridgewater, NJ). Preclinical studies demonstrated high affinity binding of IMC-A12 to IGF-1R and inhibition of the IGF-1R signaling pathway; in addition, both single agent activity as well as additive and synergistic effects with cytotoxic agents and targeted therapies like cetuximab and mTOR inhibitors were observed (14). Two phase II studies have been performed by ECOG investigating IMC-A12 activity in lung cancer patients. The ECOG 4508 study (15) randomized patients with metastatic NSCLC that were ineligible for bevacizumab to treatment with carboplatin and paclitaxel combined with either cetuximab weekly, IMC-A12 every two weeks, or both. The trial was terminated early for safety concerns related to excessive 30-day mortality with the four-drug regimen, after only 129 patients of planned 180 patients were enrolled. The median PFS was similar between the arms: 3.4 months in cetuximab arm, 4.3 months in IMC-A12 arm, and 4.1 months in combination arm. OS was also comparable in all treatment groups: 11.7 months cetuximab arm, 9.6 months IMC-A12 arm, and 8.4 months in combination arm. There were 13 deaths on treatment, including 9 patients who died within 1 month of initiation of study drug. There were higher rates of neutropenia, hyperglycemia, and thromboembolic events in the treatment arms that included IMC-A12. However, 6 of the 13 deaths occurred in patients who did not receive IMC-A12 so the high mortality seen was not solely due to IGF-1R inhibition, and could have resulted from pre-existing medical conditions. Studies utilizing IMC-A12 with other agents in lung cancer have proceeded without excessive toxicities.
IMC-A12 is also being studied in patients with small cell lung cancer. The ECOG 1508 study enrolled patients with extensive stage small lung cancer and randomized them to treatment in one of three arms: standard cisplatin and etoposide for 4 cycles, cisplatin and etoposide in combination with GDC-0449, an oral Hedgehog inhibitor, for 4 cycles with continuation of GDC-0449 as maintenance therapy, or cisplatin and etoposide with IMC-A12 on days 1, 8, 15 with IMC-A12 maintenance therapy until disease progression. The primary endpoint of the study is PFS; the study has completed accrual and the results are anticipated to be reported soon.
IMC-A12 has also been studied in combination with EGFR inhibitors. Results from a phase I study of erlotinib in combination with IMC-A12 were recently published (16). This trial examined the safety of erlotinib 150 mg daily combined with 3 different doses of IMC-A12: 6 mg/kg weekly or 5 mg/kg weekly given on 28 day cycle or 15 mg/kg every 21 days. Eighteen patients were treated and the most frequent toxicities seen were fatigue, rash, and diarrhea. Four patients in the 6 mg/kg dose cohort had DLTs including 3 patients with grade 3 fatigue and 1 patient with grade 3 acneiform rash. The 5 mg/kg dose was declared as the maximum tolerated dose for the weekly schedule. Five patients had stabilization of disease as best response while the remaining 13 patients progressed on study. The median PFS was 39 days (range, 21-432 days), with no significant differences in efficacy seen between the three dose cohorts. Three patients with activating EGFR mutations had a median duration on study of 217 days compared to 37 days in the wild-type EGFR group. Only 13 patients had serum available for biomarker analysis. There was a non-significant trend towards benefit in patients with the highest quartile of free IGF-1. This study was terminated from a planned expansion into phase II study due to lack of robust efficacy with the combination regimen (12). The strategy of combining EGFR tyrosine kinase inhibitors with IGF-1R, though promising from preclinical studies, has failed to translate into meaningful improvement in the treatment of unselected NSCLC patients.
Two other studies utilizing IMC-A12 in NSCLC patients are currently ongoing. A phase II study by ECOG evaluates carboplatin/paclitaxel/bevacizumab with or without IMC-A12 in patients with metastatic or recurrent NSCLC in the frontline setting (ClinicalTrials.gov NCT 00955305). The primary endpoint will be PFS with secondary endpoints of OS, ORR, and toxicity. The other open study is the JAEM trial sponsored by Eli Lilly (Indianapolis, Indiana), a phase II trial randomizing patients with metastatic NSCLC with non-squamous histology to treatment with either cisplatin/pemetrexed/IMC-A12 or cisplatin/pemetrexed (ClinicalTrials.gov NCT 01232452). The primary endpoint will be PFS with secondary outcomes including ORR, OS, duration of response, time to progression, change in tumor size, and quality of life assessment in a total of 220 patients. The study will have a major emphasis on biomarker assessment.
MK-0646
MK-0646 (Dalotuzumab) is another monoclonal antibody against IGF-1R that was developed by Merck (Whitehouse Station, NJ). Phase I data were published last year examining the safety of MK-0646 in patients with tumors that expressed IGF-1R as determined by immunohistochemistry (IHC) (17). Eighty patients were treated with breast and colon cancer being the most common tumor-types. The drug was well tolerated with the most frequent toxicities of hyperglycemia, asthenia, chills, back pain, and aspartate aminotransferase elevation. There were 3 patients with grade 3 or higher toxicity, including tumor pain, hyperglycemia, and a biopsy proven leukocytoclastic vasculitis, which resolved once study drug was discontinued. MK-0646 dose was escalated from 1.25 to 20 mg/kg in a total of 6 dose cohorts without an MTD being achieved. Pharmacokinetic and pharmacodynamics data was also collected; various protein levels were assessed by IHC and quantified by histochemical scores (H-scores). In 33 matched pairs of baseline and on-treatment tumor samples and 69 matched pairs of baseline and on-treatment skin biopsy samples, there was decrease in IGF-1R levels in both tumor (P=0.02) and skin (P=0.04) after 3 weeks of MK-0646 treatment. Reduced expression of proteins regulated by IGF-1R signaling, such as EGFR and phosphorylated MAP kinase, was also seen. In the 76 patients who had evaluable responses by RECIST criteria, 6 patients had stable disease, including 2 patients with Ewing’s sarcoma. It is interesting that although all the patients were selected for the study by the presence of IGF-1R expression by IHC, this did not predict treatment response with MK-0646 monotherapy.
Subsequently, a phase I/II study of erlotinib in combination with MK-0646 in unselected patients with advanced or metastatic NSCLC that has progressed after first-line therapy was conducted (ClinicalTrials.gov NCT 00654420). Another study that has completed accrual is a phase Ib study of erlotinib and MK-0646 to determine imaging and molecular determinants of response. This study utilized positron emission tomography (PET) response at weeks 1 and 3 to guide therapy with PFS and OS as secondary endpoints. Patients with locally advanced or metastatic NSCLC that progressed on 1 or 2 prior chemotherapy regimens were treated with erlotinib for one week. If there was a decrease in FDG uptake seen on PET, the patients continued on erlotinib. If there was no PET response, the patients were continued on erlotinib in combination with MK-0646 until disease progression. Patients were allowed to crossover from erlotinib to combination arm at time of disease progression. Results for this study have not yet been reported.
IGF-1R inhibition in other malignancies
To date, the efficacy of IGF-1R inhibition in NSCLC has been disappointing. There have been multiple studies of IGF-1R blockade in other tumor types, including colon cancer, pancreatic cancer, and breast cancer that have shown limited activity. The most promising data have been seen in Ewing’s sarcoma. Ewing’s sarcoma is defined by the presence of the EWS/FL-1 fusion gene, which results in malignant transformation in an IGF-1R dependent manner (18). Inhibition of IGF-1R inhibits Ewing’s sarcoma growth in tumor xenograft models (19). Based on these preclinical data, the phase I study of figitumumab included expansion cohorts for patients with refractory advanced sarcomas and specifically for Ewing’s sarcoma (20). There were 29 sarcoma patients with 16 with Ewing’s sarcoma. Of the 28 patients with response data, 2 patients with Ewing’s sarcoma had objective responses, including 1 complete response. Eight patients achieved stable disease for at least 4 months.
Further studies of IGF-1R monoclonal antibodies in Ewing’s sarcoma have also failed to show impressive single agent efficacy. The phase I/II study of figitumumab enrolled 31 patients with sarcoma to 2 dose escalation cohorts (21). There were 107 patients with Ewing’s sarcoma enrolled in the phase II portion of the study; 15 of 106 evaluable patients had partial response (ORR 14.2%) and 25 achieved stable disease with a median OS of 8.9 months. Again, free IGF-1 levels predicted patients that benefited most from IGF-1R inhibition: patients with baseline IGF-1 higher than 0.65 ng/mL had median OS of 10.4 months (P<0.001) compared to OS of 3.6 months in those with lower levels. A phase II study of R1507 enrolled 115 patients with refractory or recurrent Ewing’s sarcoma to treatment with R1507 dosed at 9 mg/kg weekly; however, the study was amended after ongoing PK studies demonstrated that higher peak serum concentrations attained with 27 mg/kg every 3 weeks resulted in increased tumor shrinkage in a xenograft model (22). A total of 109 patients were enrolled to the weekly dose cohort and 6 patients to the higher dose cohort. The objective response rate was 10% with a median duration of response of 29 weeks and median OS of 7.6 months. Treatment was well tolerated with most common grade 3 or 4 AEs of pain, anemia, thrombocytopenia, asthenia, and hyperglycemia (3 patients). Factors found to predict survival in multivariate analysis included bone primary tumor, Karnofsky performance status of at least 90%, total IGF-1 level above 110 ng/mL, and higher percentage increase in total IGF-1 from baseline to week 6. Similarly, IMC-A12 was investigated in a phase I/II study of patients with refractory solid tumors: the recommended phase II dose was defined at 9 mg/kg (23). In 30 patients treated with Ewing’s sarcoma, only 3 patients had partial response with single agent IMC-A12. Again despite promising preclinical data, IGF-1R inhibition with monoclonal antibodies has not resulted in significant single agent activity in Ewing’s sarcoma.
Is there a future for IGF-1R targeting in oncology?
Despite promising preclinical data and strong rationale for targeting IGF-1R, clinical efficacy has been disappointing in multiple studies, in lung cancer and other diseases. There are multiple potential reasons behind the failure of IGF-1R inhibition to live up to its promise. First, no study has studied the effects of IGF-1R inhibitors on the receptor at the level of the tumor and subsequent downstream signals. Defining specificity of a targeted agent against its target in patients is challenging, as often tumor tissue is not easily accessible for multiple biopsies to test IGF-1R expression for baseline and post-treatment levels. Nonetheless, this is an integral determination to make to assess if a targeted therapy fails because it does not truly achieve the desired effects on its intended target or because inhibition of the target by itself was not adequate to induce disease response.
However, the presence of IGF-1R expression may not be enough to predict response to IGF-1R inhibition, as was seen in the MK-0646 phase I study (17). Most tumors are not dependent on only one aberrant signaling pathway for tumor growth. There are multiple downstream signaling pathways that are activated by IGF-1R signaling; any of these pathways can become constitutively activated to overcome loss of IGF-1R signaling. For example, mTOR signaling has been shown to be activated in breast cancer cell lines resistant to IGF-1R inhibition; this could be overcome by treatment with the mTOR inhibitor everolimus (24). This combination has been studied in phase I studies and shown to have promising activity, particularly in patients with Ewing’s sarcoma and adrenocortical carcinoma (25-27). Given the genetic complexity of most tumors, particularly lung cancer, targeting of multiple signaling pathways will likely be required to result in therapeutic efficacy.
The insulin receptor (IR) also signals through the PI3K-AKT pathway. IR signaling is up-regulated when IGF-1R expression is decreased by treatment with monoclonal antibodies (28). OSI Pharmaceuticals (Melville, NY) is developing orally bioavailable dual IGF-1R and IR inhibitor, OSI-906, which has been shown to have efficacy in preclinical cell line and tumor xenograft models with activated IR and IGF-1R signaling (29). This compound binds reversibly with the ATP binding cleft of the catalytic domains of both receptors and can bind both the active and inactive forms of the receptor. This dual targeting may be another way to make IGF-1R blockade a more effective strategy.
One of the major lessons to be learnt from the development of IGF-1R inhibitors in NSCLC is the lack of efforts to develop patient selection methods as part of early phase studies. The negative results of large phase III studies quickly quelled the enthusiasm for development of these agents and halted further investment into biomarker discovery. However, as can be seen from the studies described in this article, elevated baseline serum IGF-1 levels have shown near consistent correlation with improved efficacy with IGF-1R antagonists. These observations were noted in phase I and II studies. However, the phase III studies were conducted in unselected patients even before the results of phase I and II studies could be carefully analyzed for predictive biomarkers. For example, figitumumab was immediately pushed into phase III studies with combinations of chemotherapy and with erlotinib without waiting for biomarker analysis from the earlier phases of study. Unfortunately, both strategies utilizing figitumumab have been proven futile. The pressure to bring drugs to patients early, has no doubt contributed to the conduct of phase III studies with limited supporting data. Unfortunately, too often, potentially useful drugs have been discarded because of an accelerated development path without careful biomarker discovery efforts. We believe that the biological data that link IGF-1R pathway activation to cancer are compelling and the rationale to inhibit IGF-1R is sufficiently strong. IGF-1R inhibition is likely still a viable target in lung cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- World Health Organization. Cancer Fact Sheet 2012 (cited 2012 September 28). Available online: http://www.who.int/ mediacentre/factsheets/fs297/en/
- Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer--report of a Canadian multicenter randomized trial. J Clin Oncol 1988;6:633-41.
- Tao Y, Pinzi V, Bourhis J, et al. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nat Clin Pract Oncol 2007;4:591-602.
- Sell C, Rubini M, Rubin R, et al. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A 1993;90:11217-21.
- Yang XF, Beamer WG, Huynh H, et al. Reduced growth of human breast cancer xenografts in hosts homozygous for the lit mutation. Cancer Res 1996;56:1509-11.
- Frankel SK, Moats-Staats BM, Cool CD, et al. Human insulin-like growth factor-IA expression in transgenic mice promotes adenomatous hyperplasia but not pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2005;288:L805-12.
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915-28.
- Karp DD, Paz-Ares LG, Novello S, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol 2009;27:2516-22.
- Jassem J, Langer CJ, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28:abstr 7500.
- Gualberto A, Hixon ML, Karp DD, et al. Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab. Br J Cancer 2011;104:68-74.
- Pfizer. Pfizer Discontinues A Phase 3 Study Of Figitumumab In Previously Treated Patients With Advanced Non-Small Cell Lung Cancer. 2010 (cited 2012 November 1); Available online: http://www.pfizer.com/ news/press_releases/pfizer_press_release_archive.jsp?guid= 20100311006790en&source=2010&page=11
- Ramalingam SS, Spigel DR, Chen D, et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J Clin Oncol 2011;29:4574-80.
- Habben K, Delmar P, Brownstein CM, et al. Investigation of predictive biomarkers for R1507, an anti-IGF1R antibody, in patients with advanced non-small cell lung cancer with progression after first-line chemotherapy. J Clin Oncol 2011;29:abstr 7584.
- Rowinsky EK, Youssoufian H, Tonra JR, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res 2007;13:5549s-5555s.
- Hanna NH, Dahlberg SE, Kolesar J, et al. Three-arm randomized phase II study of carboplatin (C) and paclitaxel (P) in combination with cetuximab (CET), IMC-A12, or both for advanced non-small cell lung cancer (NSCLC) patients who will not receive bevacizumab-based therapy: An Eastern Cooperative Oncology Group (ECOG) study (E4508). J Clin Oncol 2012;30:abstr 7516.
- Weickhardt A, Doebele R, Oton A, et al. A phase I/II study of erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (cixutumumab) in patients with advanced non-small cell lung cancer. J Thorac Oncol 2012;7:419-26.
- Atzori F, Tabernero J, Cervantes A, et al. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2011;17:6304-12.
- Toretsky JA, Kalebic T, Blakesley V, et al. The insulinlike growth factor-I receptor is required for EWS/ FLI-1 transformation of fibroblasts. J Biol Chem 1997;272:30822-7.
- Scotlandi K, Benini S, Nanni P, et al. Blockage of insulinlike growth factor-I receptor inhibits the growth of Ewing’s sarcoma in athymic mice. Cancer Res 1998;58:4127-31.
- Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti- IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol 2010;11:129-35.
- Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 2011;29:4534-40.
- Pappo AS, Patel SR, Crowley J, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol 2011;29:4541-7.
- Malempati S, Weigel B, Ingle AM, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:256-62.
- Ekyalongo RC, Mukohara T, Kataoka Y, et al. Mechanisms of acquired resistance to insulin-like growth factor 1 receptor inhibitor in MCF-7 breast cancer cell line. Invest New Drugs 2012. [Epub ahead of print].
- Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res 2011;17:871-9.
- Naing A, Kurzrock R, Burger A, et al. Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer. Clin Cancer Res 2011;17:6052-60.
- Naing A, LoRusso P, Fu S, et al. Insulin growth factorreceptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res 2012;18:2625-31.
- Buck E, Gokhale PC, Koujak S, et al. Compensatory insulin receptor (IR) activation on inhibition of insulinlike growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther 2010;9:2652-64.
- Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem 2009;1:1153-71.


