Treatment for small cell lung cancer, where are we now?—a review
Introduction
Lung cancer is the main cause of cancer-related death worldwide, and the second in incidence in both genders; nevertheless, the small cell lung cancer (SCLC) subtype covers only 13% to 15% of total lung cancer diagnoses. A decrease in its incidence has been seen in the last 20 years, probably related to the decrease in tobacco use in occidental countries (1), as well as to the recognition of other subtype: large cell neuroendocrine carcinoma, which before 1990 was considered to be SCLC. The worldwide estimate of SCLC for 2015 is 260,000 new cases, with over 11,000 in Europe (2). The 5-year mortality is expected to be about 90%.
SCLC is a highly aggressive, undifferentiated neoplasia that originates from the precursors of neuroendocrine cells. It is characterized by a high proliferation rate and early metastasis. Although it is very sensitive to cytotoxic therapy and ionizing radiation, it has been observed to develop early resistance to conventional treatments, therefore showing early progression and lack of sensitivity to further pharmacological treatment.
The main cause of SCLC is tobacco use [at least 95% of patients have positive smoking history (3)]. Quitting smoking has been related not only to a reduction in the incidence of the disease, but also to a significant reduction in the risk of mortality—about 50% in early stages (4). Other environmental and occupational carcinogens have been related to SCLC, but the strongest evidence of the increase of SCLC is exposure to chloromethyl ether (used in the chemical manufacturer industry) and high radon levels, to which uranium miners are exposed (5).
The Veterans’ Affairs Administration Lung Cancer Study Group (VALG) proposed in 1957 a classification system based on extension of the disease rather than on the traditional TNM system used for most malignant diseases. This classification recognizes only two stages, limited disease (LD)—defined as tumor confined to one hemithorax with or without loco-regional adenopathies that could be included in a single radiation field—and extensive disease (ED), described as having escaped from the previous stage parameters, including the presence of hematogenous metastasis and malignant pleural effusion (6).
A retrospective survival analysis of 8,088 SCLC patients by the International Association for the Study of Lung Cancer (IASLC) demonstrated that patients classified as ED due to the presence of malignant pleural effusion had an intermediate prognosis between LD and ED. Therefore, it was concluded that this classification was not adequate for all patients. Nowadays it is considered that the TNM classification proposed by AJCC’s 7th edition for non-small cell lung cancer (NSCLC) (7) should be used for SCLC, since it is more descriptive and precise. Another point that stands out of this analysis is the need to prove the malignant nature of pleural effusions by cytology (8). The former term LD now corresponds to tumors T1–3N0–3M0, while ED refers to patients with metastatic disease. It is also accepted that there is a group with better prognosis compared to LD, known as “very limited stage (LS)” that includes T1–2N0–1M0 tumors. At initial diagnosis, only 30% of SCLC patients present with LD, and the rest with metastatic disease.
Median and overall survival (OS) at 2 years in patients with localized disease varies from 15 to 20 months and 20% to 40% respectively (9). Patients with advanced disease have on average disease free survival (DFS) of 5.5 months and median survival of less than 10 months, although ranges of response to different treatments approach 70% (10).
Treatment overview
The treatment of patients with SCLC is complicated, since they usually present with multiple important comorbidities secondary to tobacco use such as chronic obstructive pulmonary disease, ischemic cardiopathy and hypertension, thus deteriorating their functional status. In addition, SCLC is highly aggressive and is accompanied in general by significant weight loss, fatigue and symptoms related to bulky intrathoracic disease and/or metastasis that contribute to the patients’ frailty and obstruct optimal oncologic treatment.
In general, patients with LD should be offered concomitant chemo-radiation and those with ED, palliative chemotherapy. Nevertheless, patients with very LS may benefit from surgical treatment sometimes.
Chemotherapy is based on cisplatin, as for NSCLC. Patients receiving platinum-based treatment may be empirically divided into refractory, resistant and sensitive to platinum based on their response to first-line chemotherapy as well as their progression free interval (PFI). Refractory patents are those with progressive disease during first-line treatment. Those showing an initial response but progressing within the first 3 months after treatment completion is defined as resistant. Finally, those patients with a PFI longer than 3 months after the end of the first-line treatment are considered as sensitive. When this last group of patients relapses, it is globally accepted to receive the same chemotherapy as for first-line treatment. For the former two groups, who have worse prognosis, treatment with anthracyclines or topotecan is recommended (11).
Treatment of very LS (stage I disease)
Approximately only 5% of SCLC patients present as T1–2N0–1M0; these have shown to have a more favorable long term prognosis, with a 5-year OS of 50% (12).
LS SCLC is a potentially curable disease when it is aggressively and rapidly treated with concomitant chemo-radiation. Surgery is rarely indicated, since most patients present in advanced stages or with extensive ganglionar involvement. Nevertheless, it has been seen in retrospective studies that in patients with very LS or stage I (T1–2N0M0) that undergo lobectomy, and with confirmed lack of mediastinal and supraclavicular involvement, long-term OS is 40–60% (13,14). NCCN guidelines consider that segmentary or wedge excisions are not adequate and that lobectomy with mediastinal node resection should be performed in order to obtain maximum benefit of the surgical treatment approach. On the other hand, it was demonstrated that patients with node involvement do not benefit from excisional treatment, and therefore this modality should not be offered to patients with N1–3 disease (15).
In cases where pathologic analysis of mediastinal nodes sampling is positive for malignant diseases (N1 or N2) and a complete lymphadenectomy has not been performed, post-operative mediastinal radiotherapy should be considered. It is also mandatory to offer four cycles of adjuvant chemotherapy in patients with successfully treated stage I disease (N0) (16,17).
Treatment of LS (stages I, II and III)
The combination of chemotherapy and thoracic irradiation is preferred to unimodal chemotherapy in the management of limited SCLC (18,19). Two meta-analyses have demonstrated that combining chemotherapy with thoracic irradiation significantly diminishes local relapse and improves OS (20,21). The first meta-analysis published by Pignon et al. (20), reviewed data of 2,140 patients from 13 studies and found a better 3-year OS for patients treated with chemo-radiotherapy when compared to chemotherapy only [14.3% vs. 8.9%, respectively; HR 0.86, 95% confidence interval (CI): 0.78–0.94, P=0.001]. In the second meta-analysis, Warde and Payne (21) combined the data of 11 randomized studies with or without chemo-radiotherapy and found that bimodal treatment offered a better local control at 2 years when compared to chemotherapy alone (34.1% vs. 16.5%, respectively). A significant improvement in tumor control in the thorax in patients receiving thoracic radiation therapy (+25.3%, 95% CI: 16.5–34.1%). The OS at 2 years was 5.4% greater in patients treated with chemo-radiation (P<0.05). However, the best order and modality to combine these treatments remains to be established.
Multiple studies have demonstrated that concurrent chemo-radiation, as well as early starting of radiotherapy (after the first or second cycles), produce better disease control than the sequential modality. Murray et al. (22) studied patients treated with cyclophosphamide, doxorubicin and vincristine, alternating with etoposide and cisplatin. Patients were distributed in two groups, those starting radiotherapy on the third week of treatment and those receiving it on week 15. Patients from the early radiation group had a significant improvement in progression-free survival (PFS) (P=0.036) and OS (P=0.008). The phase III study of the Japan Clinical Oncology Group (JCOG 9104) (23), compared concurrent chemo-radiotherapy to sequential treatment (chemotherapy followed by radiotherapy) in patients treated with LD SCLC. All patients received cisplatin and etoposide. Those receiving concurrent treatment had better OS at 2, 3 and 5 years vs. those in the sequential group (OS at 2 years 54.4% vs. 35.1%; at 3 years 29.8% vs. 20.2%; at 5 years 23.7% vs. 18.3%) but these differences were not statistically significant (P=0.097). Another meta-analysis collected data from 1985 to 2002 (24) and demonstrated a slight but significant difference in OS at 2 years with the use of early-start radiotherapy. This improvement was more evident with hyper-fractionation and platinum-based chemotherapy schemes. A meta-analysis (25) evaluated seven randomized studies where radiotherapy was stared early with platinum-based chemotherapy and found a better OS at 2 and 5 years when started within the first 30 days after chemotherapy.
The inferiority of sequential treatment compared to concurrent bimodal treatment proves the development of chemo-resistant clones, which are also radiation-resistant and produce a quick repopulation of the tumor (26). Additionally, another study by the Gyeongsang National University of Korea (27) reported in 2013 that concurrent chemo-radiation was superior to sequential treatment in terms of OS, with an acceptable toxicity profile. Based on the results of all the studies, concurrent treatment is currently the standard modality for LD SCLC (28).
The radiobiology argument in favor of this recommendation is that radiation can effectively remove chemo-resistant cells before they spread outside the chest. The simultaneous start of radiotherapy and chemotherapy has several disadvantages. First, chemosensitivity of the tumor cannot be verified in vivo, on the other hand, a higher volume has to be radiated, which can generate increased toxicity and complications, especially in patients with risk of poor pulmonary reserve. Complications include radiation pneumonitis, bone marrow suppression and radioepithelitis. It has also been documented that tolerance to chemotherapy decreases if initiated simultaneously with radiotherapy, so slow administration of cytotoxics reduces the long-term gain in relapse-free survival and distant relapse decrease. Because of these results, in general, early onset of radiation therapy is preferred after cycle 1 or 2 of chemotherapy (22,24). Nevertheless, a phase III trial addressing temporality (early vs. late radiation) showed that late radiation-chemotherapy (started at the third cycle) was no inferior to early start (with the first cycle of chemotherapy) in terms of complete response (36% vs. 38%). Regarding toxicity, the late radiation group had less grade 3–4 neutropenia (29).
There are still important questions without a definite answer, such as what is the optimal radiation dose, fractionation and the ideal starting time. It is accepted with reasonable evidence that the standard of care involves the concurrent administration of radiotherapy with chemotherapy scheme PE (cisplatin 60 mg/m2 IV on day 1 and etoposide 120 mg/m2 IV days 1 to 3) in a total of 4–6 cycles (30,31), with a maximum benefit when started early (after the first or second cycle of chemotherapy) and a manageable toxicity in the concurrent scheme. Some studies suggest that the best form of radiotherapy is hyperfractionation, such as the study from the Intergroup, which directly compared a group with a single fraction of radiation a day versus. Two fractions (hyperfractionation), i.e., 45 Gy/25 fractions/5 weeks vs. 45 Gy/30 fractions/3 weeks with concurrent administration of cisplatin and etoposide. Initial analysis showed excellent results with median survival of 20 months and 40% 2-year OS, with a short follow, 5 years. A significant gain in survival for the hyperfractionation mode compared with the group who received conventional fractionation group once a day (26% vs. 16%) was achieved. The main difference appears to be in temporary acute toxicity, with an increase in grade 3 esophagitis in the group treated twice daily (27% vs. 11%, P<0.001) (32).
The RTOG 0239 study (33) reported in phase I and II the concurrent chemo-radiotherapy with a “boost” approach. This scheme initially consisted of larger radiation field covering the primary tumor in daily fractions of 1.8 Gy, with a total of 61.2 Gy in 5 weeks and a second fraction of 1.8 Gy at a smaller field during the last 9 days of treatment, standard chemotherapy with cisplatin and etoposide. The locoregional tumor control at 2 years was 80%, although survival achieved in the same time range was only 37%, a non-significant gain when compared with other forms of radiation. However, presentation of grade 3 esophagitis was significantly improved and occurred only in 18% of patients vs. 27% in Intergroup 0096 study.
A phase III trial (CALGB 30610/RTOG 0538) (34) evaluating three different radiotherapy approaches in patients with LD SCLC is currently ongoing. The patients enrolled in this study received either 70 Gy (2 Gy once daily over 7 weeks) or 61.2 Gy (1.8 Gy once daily for 16 days followed by 1.8 Gy twice daily for 9 days). The aim is to improve median and 2-year OS compared to that from patients receiving 45 Gy (1.5 Gy twice daily over 3 weeks). The results are highly awaited.
Prophylactic radiotherapy to the central nervous system (CNS) [prophylactic cerebral irradiation (PCI)]
The CNS is a common site of metastasis in patients with SCLC. About 25% present at initial diagnosis with brain metastases (35). Of the patients who achieve a complete response to initial treatment, approximately 45% will present with CNS metastases as the only site of recurrence at 2 years (36,37). The presence of CNS disease significantly impairs functional status of patients and increases the need for hospitalizations (38). Therefore, prevention of relapse to CNS is an important component of treatment of patients with SCLC.
After finishing chemo-radiotherapy in patients with adequate systemic control without evidence of metastatic disease to the CNS, PCI should be considered. Multiple studies have been done trying to assess the role of PCI. Unfortunately, most are small, heterogeneous studies with, not surprisingly, wide variability in their findings (39). Despite this, all show a significant decrease in the incidence of brain metastases. Two separate meta-analyses concluded that PCI reduces the incidence of CNS metastases between 52–54% and improves survival from 16% to 18% in those patients who achieved a complete response (40,41). A meta-analysis published in 1999 (41), involving 987 patients in remission reported a 5.4% increase in overall 3-year survival; as well as an increased rate of DFS (95% CI: 0.65–0.86; P<0.001) in patients who received PCI. As expected, a decreased incidence of brain metastasis was observed (relative risk of 0.46; 95% CI: 0.38–0.57; P<0.001).
The optimal dose of each fraction of radiation is still unclear. A randomized trial comparing standard dose of radiotherapy 25 Gy in 10 fractions to the PCI against high dose (36 Gy in 18 fractions once a day or 36 Gy in 24 fractions using 1.5 Gy twice daily) in 720 patients with LD SCLC who had complete response to treatment with chemo-radiotherapy showed no significant reduction in the incidence of brain metastases in the high dose group, but did find a significant increase in mortality (42). Currently the accepted standard is 25 Gy in 10 fractions to the PCI.
One of the biggest issues with PCI is long-term neurotoxicity. The frequency and severity of chronic toxicities associated with PCI are still unclear. Because patients undergoing PCI have an improvement in OS, they are more likely to develop chronic neurotoxicity. In a retrospective study of 98 patients who received PCI a significant improvement in mean quality time without symptoms and toxicity (Q-TWiST) (43) was observed. In a similar analysis, qualitatively life expectancy adjusted quality-adjusted life expectancy (QALE) was evaluated. PCI offers a benefit over no-PCI (QALE =4.31 and 3.7 for mild toxicity, 4.09 and 3.70 respectively for severe toxicity) (44). The results of these analyses suggested benefit of PCI despite chronic toxicity. However, it is necessary to design clinical trials with the specific aim to adequately answer the question of benefit vs. neurotoxicity and quality of life in patients receiving PCI.
Nowadays, the recommendation is to offer PCI to all LD-SCLC patients with good PS due to the evidence of the benefit of this therapy.
Treatment of ED (stage IV)
Advanced stages constitute an incurable form of disease and platinum-based chemotherapy remains the cornerstone of treatment with the aim of palliation of symptoms and increase survival. In general, treatment has an objective response of 60–75%, achieving up to 10% of radiological complete response. Although chemotherapy meets the objective of prolonging survival, relapse is the rule and only 5% of patients are alive 2 years after diagnosis.
The standard treatment of ED is chemotherapy as the only treatment modality, usually with cisplatin or carboplatin plus etoposide for up to six cycles, followed by active surveillance (45). Even patients with impaired functional status, Eastern Cooperative Oncology Group (ECOG) 3 or 4, should be considered for systemic chemotherapy because of potential responses of up to 75% and clinical improvement of symptoms secondary to the disease within a few days.
OS in patients with SCLC has changed little since the late 70s (46). Between 1973 and 2002, the 2-year survival for patients with LD improved from 15% to 22%, but the improvement in ED in the same period has been marginal, from 3.4% to 5.6% (47).
Historically SCLC patients not receiving therapy had a very poor prognosis with a median survival of seven weeks for those with ED and 14 weeks for patients with LD (48). The initial clinical studies with chemotherapy with one drug showed surprisingly that it was a very chemosensitive tumor, resulting in a long list of active drugs including platinum compounds (cisplatin and carboplatin), camptothecins (topotecan, irinotecan), podophyllotoxins (etoposide, teniposide), anthracyclines (doxorrubina, epirubicin), alkylating agents (cyclophosphamide, ifosfamide), taxanes (paclitaxel, docetaxel) and vincristine, however responses to monodrug-based therapy were short-lived with low complete response rates, so the next step was to test effective combinations against SCLC.
Interestingly, intrathoracic control remains as one of the main sites of progression [about 90% of the patients at one year of diagnosis (49)] and some studies suggesting it may be beneficial for the patients in terms of local control and OS (50-52). The findings of a recent phase III trial by Slotman showed that patients with ED-SCLC have and improved OS at 2 years (13% vs. 3%, P=0.04) added to the PFS benefit at 6 months (24% vs. 7%, P=0.001) with an acceptable toxicity profile (53).
Schemes with alkylating agents
Studies performed in the 70s found that combined chemotherapy based on cyclophosphamide, doxorubicin/epirubicin and vincristine [CA(E)V] was effective in SCLC (54,55). This multidrug therapy based on alkylating agents significantly improved OS when compared with single drug-based treatments. In the patients with response ranges between 60% to 80% complete response rate of 15–25% and median survival time of 7–10 months were obtained. Unfortunately all ED patients eventually relapse with refractory disease (Table 1).
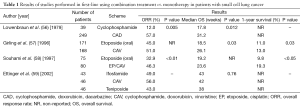
Full table
Etoposide and cisplatin (EP) regimen
In the 80s, combinations of regimens containing EP without alkylating agents were evaluated in patients with SCLC. The combination of EP obtained similar to that of the old schemes with alkylating results, but with a more favorable toxicity profile (45). More recently, a phase III study compared CEV EP (cyclophosphamide, epirubicin, and vincristine) and reported a significant improvement in OS in patients receiving EP (10.2 vs. 7.8 months, P=0.0004), which was most evident in the LS group of patients with SCLC (30). As a result of this study the EP scheme became the standard treatment for patients with SCLC. These results for EP have been confirmed in two meta-analyses, which show a modest but significant improvement in OS and a better toxicity profile, especially in patients receiving concurrent radiotherapy (31,60). The meta-analysis published by Pujol et al. (31) analyzed 19 randomized studies with a total of 4,054 patients, demonstrating a reduction in risk of death at 6 months and 1 year of 13% and 20%, respectively (OR 0.87, 95% CI: 0.75–0.98, P=0.03, and 0.80, 95% CI: 0.69–0.93, P=0.002) in patients who received a treatment regimen based on platinum. The second meta-analysis by Mascaux et al. (60), divided its analysis into four groups: group I, studies that included cisplatin-based regimens without etoposide; group II, 17 studies that included schemes without cisplatin etoposide; group III, 9 studies comparing a regimen including the combination of EP against other drug regimens; and group IV which compared 9 studies with monodrug EP against etoposide. Ninety five percent HRs were: 0.70 (0.41–1.21) for group I, 0.72 (0.67–0.78) in group II, 0.57 (0.51–0.64) group III, and finally 0.74 (0.66–0.83) for group IV. The benefit was seen in the groups that included the main agent cisplatin (HR =0.61; 95% CI: 0.57–0.66), as well as those involving etoposide (HR =0.65; 95% CI: 0.61–0.69).
Carboplatin
Carboplatin has been tried as a substitute for cisplatin in combination with etoposide and generally no difference was found in the ranges of response, though carboplatin is associated with less toxicity (61). Nevertheless, the COCIS meta-analysis showing no differences between cisplatin and carboplatin in terms of efficacy; the toxicity profile was quite different. Myelosuppression had a higher incidence in the Carboplatin group (mainly neutropenia, anemia and thrombocytopenia); whilst patients treated with cisplatin had significantly more non-hematologic toxicity (i.e., nausea, neurotoxicity and renal toxicity) (62). A second study evaluated long-term survival with carboplatin and concurrent radiotherapy in LD: patients had a median survival of 17.4 months and OS at 5 years of 20%, comparable to those of cisplatin (63).
Irinotecan
In Japan, in 2002 a phase III study, 9511 JCOG (64), comparing EP against irinotecan/cisplatin (irinotecan at a dose of 60 mg/m2 on days 1, 8 and 15 cycles every 3 weeks) was conducted. This study was closed early because of the significant improvement in OS for patients receiving irinotecan for 12.8 months compared with the group of patients who received etoposide for 9.4 months, with a significant increase in response of 84% vs. 68%. Although this scheme was adopted as a standard treatment in Japan, these results have not been corroborated in Western populations. There have been three phase III studies using irinotecan and cisplatin, with small modifications. In the first, the irinotecan dose was 65 mg/m2 on days 1 and 8 every 3 weeks. The 331 patients were randomized 1:2 to etoposide: irinotecan, resulting in a median survival of 10.2 months and 9.3 months etoposide to irinotecan (65). Another phase III study conducted with the same scheme as the Japanese study (66) with 651 patients showed no difference in survival (9.9 months for irinotecan and 9.1 months for etoposide P=0.71), with higher gastrointestinal toxicity with irinotecan (diarrhea) and hematologic toxicity for the etoposide arm. A third study compared carboplatin (AUC 5) combined with irinotecan at 50 mg/m2 on days 1, 8 and 15 with carboplatin/etoposide. Two hundred and sixteen patients were evaluated, with survival of 10 months in the irinotecan group and 9 months for those who received etoposide (67).
A reasonable alternative is the combination of platinum plus irinotecan in first-line ED SCLC instead of the EP regime, according to the profile of individual patient toxicity. However, evidence from three Western studies did not corroborate the impressive survival average over 12 months in the Japanese study. These conflicting results may be due to pharmacogenomic differences between Japanese and Western populations in the metabolism of irinotecan, so EP remains the standard first-line treatment for extensive stage (ES) SCLC.
Treatment duration
Two randomized studies sought to evaluate the addition of 2 more cycles of chemotherapy followed by 4 cycles of standard EP in patients with ED SCLC (46,68). Both showed that extending therapy can improve PFS but not OS. A meta-analysis evaluated 14 studies and 2,550 patients, suggesting that maintenance chemotherapy/consolidation is associated with increased survival at 1 and 2 years of 33% (69). However, only one study used what is now considered to be modern regime of chemotherapy, both induction and consolidation phase. Today maintenance cannot be recommended outside of clinical protocols.
Prophylactic cerebral irradiation (PCI)
In 2007, the pivotal study published by EORTC clearly demonstrated the utility of PCI in ED SCLC (49). This group showed that PCI reduced risk of CNS metastases at 1 year by 25% (40% of brain metastases in the control group vs. 15% in the PCI group) in patients who responded to chemotherapy. Besides the 1-year survival in the PCI group was 27% vs. only 13% in the control group. In other words, the experimental group showed a gain of 14% in 1-year survival. Recently, in another publication by Slotman (70) the average in global health (overall health status score) was 8 points higher in patients in the PCI group, on a scale from 0 to 100, at 6 weeks of treatment (P=0.018) and 3 months (P=0.055). The expected side effects of major PCI were fatigue and alopecia. Other quality of life aspects, such as emotional and cognitive function, appear to have a limited impact. From this information it is now accepted that PCI should be offered to patients with adequate response to chemotherapy in the context of ED.
Treatment of progressive disease, second-line
Although SCLC remarkably responds to initial treatment, most patients will relapse with relatively resistant disease. These patients have a median survival of only 4 or 5 months if treated with a second- or third-line chemotherapy (71,72). Patients with progressive disease are classified based on the response they had with the primary induction therapy and its duration. Subsequent chemotherapy provides significant palliation of symptoms in many patients, but success at this point depends on the time of relapse or progression. If the interval is less than 3 months (resistant or refractory disease), most agents or regimens yield very poor response (<10%). If the time to relapse is greater than 3 months (sensitive disease) the expected response will be around 25% (15).
In particular cases which had an excellent response to first-line platinum-based and relapse that occurs after 2 months of completed induction therapy, it is recommended to retry patients with the original chemotherapy regimen (73). A meta-analysis by Owonikoko showed that patients with sensitive disease have a higher RR when compared to refractory patients (27.7% vs. 14.8%, 95% CI: 1.51–3.29; P=0.001), as well as longer OS (74).
With regard to strategies using cytotoxic second-line therapy, there is no established consensus about the best and most effective regimen but there is a tendency to use single drug agents rather than the combinations. However, there is one agent approved by the FDA specifically for this group of patients, topotecan, camptothecin. However, there are multiple phase II studies with other drugs including paclitaxel, docetaxel, irinotecan, vinorelbine, gemcitabine, ifosfamide, temozolomide and oral etoposide.
Topotecan
In a phase III study comparing intravenous topotecan with CAV regimen, both arms had similar response and survival but, as expected, the arm with the single drug was less toxic than the combination (75). In another phase III study, oral topotecan obtained better survival when compared with BSC, 26 vs. 14 weeks (P=0.014) and survival at 6 months 49% vs. 26% and the benefit was observed in all subgroups analyzed, including the refractory and platinum group with ECOG 2 (76). Topotecan has been approved by the FDA since 2007 for recurrent, resistant and sensitive platinum disease, either oral or intravenous administration because efficacy and toxicity are similar regardless of the method of administration employed (77).
There are limited data on similar effectiveness between irinotecan and topotecan but this has never been evaluated in randomized studies in the context of relapse.
Amrubicin
Amrubicin, a synthetic anthracycline, has been evaluated extensively in Japan as an alternative treatment in patients in relapse. Initial studies in North America showed promising responses, especially for platinum and topotecan-refractory patients. The first of these studies to give rise to a possible alternative to topotecan in second-line was by Onoda et al. in 2006 (78), a multicenter phase II study, which managed to recruit 60 patients with relapsed SCLC; 16 and 44 refractory patients sensitive to platinum. Amrubicin was administered on days 1–3 every 21 days and 40 mg/m2. The most surprising information with this treatment regimen was the equivalent response in both groups of patients, sensitive and refractory, 50% and 52% respectively. However, they had a higher PFS (2.6 vs. 4.2 months), OS (10.3 vs. 11.6 months) and 1-year survival (40% vs. 46%), favoring all results in the platinum-sensitive group. Hematologic toxicity with amrubicin is considerable, with grade 3 or 4 neutropenia. However, the information from several small phase II study was not conclusive of the utility of amrubicin in second-line, with a tendency to have better results than topotecan in the group of patients refractory to platinum. A phase III study recently published explored this point (79), recruiting 637 patients and reporting that amrubicin did not improve OS as second-line therapy in SCLC when compared with topotecan, with median survival of 7.5 months for the amrubicin group and 7.8 months for those receiving topotecan. The behavior of patients with refractory disease was different: amrubicin had an average survival of 6.2 months and 5.7 months for topotecan (HR =0.77, P=0.047). Amrubicin had significant results regarding the progression-free period, 4.1 vs. 3.5 months (HR, 0.802; P=0.018) and ORR 31.1% vs. 16.9% (P<0.001). The conclusion of this study is that amrubicin did not significantly improve survival of patients treated in second-line since, despite good results in the refractory population, these are not clinically significant.
Other drugs
There are several small randomized trials with drugs that have been tested in second-line, however, further evidence is only available for two of them in this context: firstly picoplatin, an organic platinum analogue specially designed for the group of patients resistant to this treatment (Table 2). The second is belotecan, a new topoisomerase I inhibitor (Table 3). Both have a very modest improvement in overall response and progression-free periods which is not been reflected in OS. Therefore, additional studies to prove or disprove its usefulness are required (11,85).

Full table

Full table
Treatment duration
Unlike first-line treatment, the optimal duration of second-line treatment has not been clearly determined but an important point to consider is the cumulative toxicity of treatment, as well as the clinical benefit and quality of life. It is globally accepted to continue treatment for two more cycles after obtaining the maximum response or until disease progression or unacceptable toxicity. Additional chemotherapy after second-line progression should only be proposed in patients who continue to have adequate performance status (15).
Conclusions
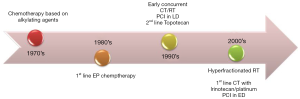
Progress in the management of patients with SCLC has been modest in the past 20 years, the main contribution to improving patient survival has been provided by concurrent radiotherapy to the chest and management PCI (Figure 1) (86). Chemotherapy with etoposide and platinum remains the standard treatment for patients in Western countries, however, it is well accepted that the Eastern countries use the couplet of irinotecan and cisplatin which has been demonstrated to be superior in the Japanese population. Although patients usually have an excellent response to chemotherapy with or without initial radiotherapy, nearly all will relapse with local or distant disease and eventually die. Tumor cells at progression are less sensitive to treatment with cytotoxics, therefore treatment selection will depend on the time of relapse or progression. Clinical and basic research should continue to identify better treatment strategies that help increase the duration and effectiveness of treatment of patients with SCLC.
Acknowledgements
The authors would like to thank Mrs Kate Williams for her assistance correcting the English version of this manuscript. D Morales-Espinosa’s work is supported with grants from IASLC’s Lung Cancer Research Fellowship Award and ESMO’s Translational Research Fellowship Award.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [PubMed]
- Globocan: Cancer Fact Sheets. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer 2012;131:1210-9. [PubMed]
- Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [PubMed]
- Churg A. Lung Biology in Health and Disease. In: Samet JM, editor. Epidemiology of Lung-Cancer. New York: Marcel Dekker Inc.,1994:413-36.
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 1973;4:31-42. [PubMed]
- Edge S, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. New York: Springer-Verlag, 2010.
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [PubMed]
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011;378:1741-55. [PubMed]
- Foster NR, Qi Y, Shi Q, et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer 2011;117:1262-71. [PubMed]
- Califano R, Abidin AZ, Peck R, et al. Management of small cell lung cancer: recent developments for optimal care. Drugs 2012;72:471-90. [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [PubMed]
- Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132-9. [PubMed]
- Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267-71. [PubMed]
- NCCN Guidelines Version 1. 2016 Small Cell Lung Cancer. Available online: http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf
- Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832-8. [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [PubMed]
- Bunn PA Jr, Lichter AS, Makuch RW, et al. Chemotherapy alone or chemotherapy with chest radiation therapy in limited stage small cell lung cancer. A prospective, randomized trial. Ann Intern Med 1987;106:655-62. [PubMed]
- Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med 1987;316:912-8. [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [PubMed]
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890-5. [PubMed]
- Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336-44. [PubMed]
- Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054-60. [PubMed]
- Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 2004;22:4837-45. [PubMed]
- Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, et al. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev 2007;33:461-73. [PubMed]
- Amini A, Byers LA, Welsh JW, et al. Progress in the management of limited-stage small cell lung cancer. Cancer 2014;120:790-8. [PubMed]
- Ha IB, Jeong BK, Jeong H, et al. Effect of early chemoradiotherapy in patients with limited stage small cell lung cancer. Radiat Oncol J 2013;31:185-90. [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006;17:543-52. [PubMed]
- Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol 2013;24:2088-92. [PubMed]
- Sundstrøm S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol 2002;20:4665-72. [PubMed]
- Pujol JL, Carestia L, Daurès JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8-15. [PubMed]
- Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265-71. [PubMed]
- Komaki R, Paulus R, Ettinger DS, et al. Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int J Radiat Oncol Biol Phys 2012;83:e531-6. [PubMed]
- Radiation Therapy Regimens in Treating Patients With Limited-Stage Small Cell Lung Cancer Receiving Cisplatin and Etoposide. Available online: https://clinicaltrials.gov/ct2/show/NCT00632853
- Hochstenbag MM, Twijnstra A, Wilmink JT, et al. Asymptomatic brain metastases (BM) in small cell lung cancer (SCLC): MR-imaging is useful at initial diagnosis. J Neurooncol 2000;48:243-8. [PubMed]
- Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87:183-90. [PubMed]
- Ball DL, Matthews JP. Prophylactic Cranial Irradiation: More Questions Than Answers. Semin Radiat Oncol 1995;5:61-68. [PubMed]
- Felletti R, Souhami RL, Spiro SG, et al. Social consequences of brain or liver relapse in small cell carcinoma of the bronchus. Radiother Oncol 1985;4:335-9. [PubMed]
- Le Péchoux C, Arriagada R. Prophylactic cranial irradiation in small cell lung cancer. Hematol Oncol Clin North Am 2004;18:355-72. [PubMed]
- Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 2001;1:5. [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [PubMed]
- Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467-74. [PubMed]
- Tai TH, Yu E, Dickof P, et al. Prophylactic cranial irradiation revisited: cost-effectiveness and quality of life in small-cell lung cancer. Int J Radiat Oncol Biol Phys 2002;52:68-74. [PubMed]
- Lee JJ, Bekele BN, Zhou X, et al. Decision analysis for prophylactic cranial irradiation for patients with small-cell lung cancer. J Clin Oncol 2006;24:3597-603. [PubMed]
- Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471-7. [PubMed]
- Hanna NH, Sandier AB, Loehrer PJ Sr, et al. Maintenance daily oral etoposide versus no further therapy following induction chemotherapy with etoposide plus ifosfamide plus cisplatin in extensive small-cell lung cancer: a Hoosier Oncology Group randomized study. Ann Oncol 2002;13:95-102. [PubMed]
- Navada S, Lai P, Schwartz AG, et al. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J Clin Oncol 2006;24:abstr 7082.
- Greco FA, Oldham RK. Clinical management of patients with small cell lung cancer. In: Greco FA, Oldham RK, Bunn PA, editors. Small Cell Lung Cancer. New York: Grune and Stratton, 1981:353-379.
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [PubMed]
- Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol 1999;17:2092-9. [PubMed]
- Zhu H, Zhou Z, Wang Y, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer 2011;117:5423-31. [PubMed]
- Giuliani ME, Atallah S, Sun A, et al. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clin Lung Cancer 2011;12:375-9. [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase III randomised controlled trial. Lancet 2015;385:36-42. [PubMed]
- Livingston RB, Moore TN, Heilbrun L, et al. Small-cell carcinoma of the lung: combined chemotherapy and radiation: a Southwest Oncology Group study. Ann Intern Med 1978;88:194-9. [PubMed]
- Feld R, Pringle JF, Evans WK, et al. Combined modality treatment of small cell carcinoma of the lung. Arch Intern Med 1981;141:469-73. [PubMed]
- Lowenbraun S, Bartolucci A, Smalley RV, et al. The superiority of combination chemotherapy over single agent chemotherapy in small cell lung carcinoma. Cancer 1979;44:406-13. [PubMed]
- Girling DJ. Comparison of oral etoposide and standard intravenous multidrug chemotherapy for small-cell lung cancer: a stopped multicentre randomised trial. Medical Research Council Lung Cancer Working Party. Lancet 1996;348:563-6. [PubMed]
- Souhami RL, Spiro SG, Rudd RM, et al. Five-day oral etoposide treatment for advanced small-cell lung cancer: randomized comparison with intravenous chemotherapy. J Natl Cancer Inst 1997;89:577-80. [PubMed]
- Ettinger DS, Finkelstein DM, Ritch PS, et al. Study of either ifosfamide or teniposide compared to a standard chemotherapy for extensive disease small cell lung cancer: an Eastern Cooperative Oncology Group randomized study (E1588). Lung Cancer 2002;37:311-8. [PubMed]
- Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23-36. [PubMed]
- Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601-7. [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [PubMed]
- Spigel DR, Hainsworth JD, Burris HA, et al. Long-term follow-up of Limited stage small cell lung cancer patients treated with carboplatin-based chemotherapy and radiotherapy by the Minnie Pearl Cancer Research Network (MPCRN). J Clin Oncol 2004;22:abstr 7222.
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [PubMed]
- Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530-5. [PubMed]
- Schmittel A, Sebastian M, Fischer von Weikersthal L, et al. A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol 2011;22:1798-804. [PubMed]
- Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593--a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:2114-22. [PubMed]
- Bozcuk H, Artac M, Ozdogan M, et al. Does maintenance/consolidation chemotherapy have a role in the management of small cell lung cancer (SCLC)? A metaanalysis of the published controlled trials. Cancer 2005;104:2650-7. [PubMed]
- Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78-84. [PubMed]
- Hurwitz JL, McCoy F, Scullin P, et al. New advances in the second-line treatment of small cell lung cancer. Oncologist 2009;14:986-94. [PubMed]
- Schneider BJ. Management of recurrent small cell lung cancer. J Natl Compr Canc Netw 2008;6:323-31. [PubMed]
- Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409-11. [PubMed]
- Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866-72. [PubMed]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67. [PubMed]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [PubMed]
- Eckardt JR, Bentsion DL, Lipatov ON, et al. Phase II study of picoplatin as second-line therapy for patients with small-cell lung cancer. J Clin Oncol 2009;27:2046-51. [PubMed]
- Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol 2006;24:5448-53. [PubMed]
- von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012-9. [PubMed]
- Treat J, Schiller J, Quoix E, et al. ZD0473 treatment in lung cancer: an overview of the clinical trial results. Eur J Cancer 2002;38 Suppl 8:S13-8. [PubMed]
- Ciuleanu T, Samarzjia M, Demidchik Y, et al. Randomized phase III study (SPEAR) of picoplatin plus best supportive care (BSC) or BSC alone in patients (pts) with SCLC refractory or progressive within 6 months after first-line platinum-based chemotherapy. J Clin Oncol 2010;28:abstr 7002.
- Rhee CK, Lee SH, Kim JS, et al. A multicenter phase II study of belotecan, a new camptothecin analogue, as a second-line therapy in patients with small cell lung cancer. Lung Cancer 2011;72:64-7. [PubMed]
- Jeong J, Cho BC, Sohn JH, et al. Belotecan for relapsing small-cell lung cancer patients initially treated with an irinotecan-containing chemotherapy: a phase II trial. Lung Cancer 2010;70:77-81. [PubMed]
- Kim GM, Kim YS, Ae Kang Y, et al. Efficacy and toxicity of belotecan for relapsed or refractory small cell lung cancer patients. J Thorac Oncol 2012;7:731-6. [PubMed]
- Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis 2013;5 Suppl 5:S565-78. [PubMed]
- Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother 2014;15:2385-96. [PubMed]


