Metachronous primary uterine cancer surgically resected during Crizotinib treatment in a ALK-rearranged advanced lung adenocarcinoma
Introduction
Rearrangements of the anaplastic lymphoma kinase (ALK) gene are present approximately in 3% to 7% of non-small-cell lung cancers (NSCLCs) (1-3). Besides EGFR mutation assessment, current guidelines recommend initial ALK testing in patients with adenocarcinoma (4-6); indeed, patients harboring ALK rearrangements show very favourable outcomes if treated with targeted agents, among which crizotinib is the first and best studied (1,3,7,8). Crizotinib, an oral small-molecule tyrosine kinase inhibitor of ALK, MET, and ROS1 kinases characterized by very good activity and tolerability, has now been included in the therapeutic algorithm of NSCLC with this type of oncogene addiction (6,8,9), although its optimal combination with other approaches, such as surgery for concomitant diseases, is unknown. In this report, a case of advanced lung adenocarcinoma harboring ALK rearrangement is described. During crizotinib treatment a major surgery for a metachronous uterine cancer was safely and successfully carried out.
Case presentation
A 71-year-old female, never smoker, due to the onset of cough and dyspnea underwent a chest X-ray and a computed tomography (CT) scan that revealed “abundant right pleural effusion and parenchymal mass at middle and inferior right lobe; the superior right lobe presented a ground-glass aspect and mediastinal lymph nodes were enlarged”.
The cytological diagnosis, obtained after pleural drainage, was lung adenocarcinoma, while the whole and scarce tissue obtained by a bronchial biopsy was entirely submitted to epidermal growth factor receptor (EGFR) mutation assessment that was at that time the only potentially druggable oncogenic addiction in first line setting.
Due to the lack of activating EGFR mutations, from December 2013 to February 2014 the patient received chemotherapy with Cisplatin plus Pemetrexed for four cycles, obtaining a radiological disease stabilization and a good clinical benefit; therefore, a maintenance Pemetrexed treatment was planned.
During maintenance chemotherapy, the re-evaluation by CT imaging confirmed the stable disease at the lung primary site; however a pelvic mass, already previously diagnosed as a uterine fibroid, showed suspicious characteristics. In addition, the pelvic mass revealed a significant 18-FDG uptake by positron emission tomography (PET) scan with maximum Standardized Uptake Value (SUVmax) of 14.9.
In December 2014, after nine cycles of maintenance therapy with Pemetrexed, the patient had a clinical worsening with cough and dyspnea. The CT scan detected the enlargement of the primary lung tumor located at lower right lobe with atelectasia of most of parenchyma and involvement of homolateral hilar structures; also the uterine mass increased with maximum diameter of 7.4 cm and broad necrotic component. The PET scan revealed high uptake of both disease sites (Figure 1).
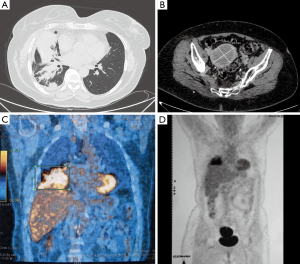
After agreement of the Lung Cancer Multidisciplinary Team of our Institution, the patient underwent a percutaneous CT-guided fine needle aspiration biopsy (FNAB) both at thoracic and uterine site, with the aim of: (I) to test the lung adenocarcinoma for the presence of ALK-rearrangement; (II) to assess the primary or metastatic origin of the uterine mass.
The cytological examination on cell blocks from lung FNAB confirmed the diagnosis of the primary lung adenocarcinoma, while the presence of ALK rearrangement was detected both by cytoplasmatic expression for Ab-anti ALK 5A4 (Leica/Novocastra) and by FISH using the Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe® (Abbott Molecular, Abbott Park, IL, USA) (Figure 2).
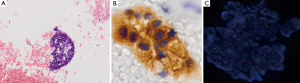
The uterine mass exhibited aggregates of small cells, with hyperchromic nuclei and scarcely represented cytoplasms, thus indicating a different histogenesis. The immunohistochemical markers confirmed the malignant nature of both neoplastic lesions and their primitivity of arising.
Therefore, the patient started a targeted therapy with the met/ALK inhibitor crizotinib, achieving a quick and marked clinical/respiratory improvement.
After two months of treatment with crizotinib, a radical hysterectomy was performed, revealing a solid endometrioid adenocarcinoma (grade 3, stage IB) (Figure 3).
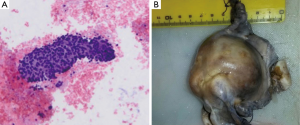
Crizotinib was stopped three days before and after surgery, that was carried out without any complication; due to the stage of uterine neoplasm, a pelvic adjuvant radiotherapy was planned.
In July 2015, after further three months of crizotinib treatment, a PET/CT scan showed marked reduction of volume and uptake of the primary right lung tumor (SUVmax of 3.2 vs. previous 10.6 value) without any radio uptake at pelvic site.
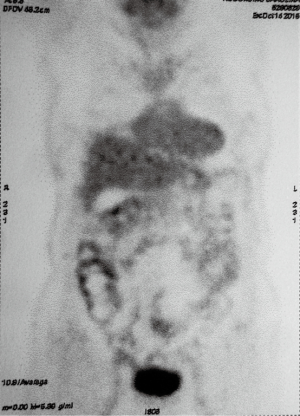
The follow up PET/CT imaging performed in October 2015 has shown the almost complete regression of the 18-FDG uptake (SUVmax of 1.3) of the primary lung tumor while a low uptake (SUVmax of 2.7) was heterogeneously distributed at pleural thickening close to middle and inferior right lobe (Figure 4) without any other site of uptake.
Two years after the initial diagnosis, the patient is asymptomatic with optimal clinical conditions and quality of life.
Discussion
This clinical case presents some reasons of interest.
First, this report describes the case of a patient with advanced lung adenocarcinoma showing progressive thoracic disease after first-line chemotherapy. Due to the very scarce tissue, the baseline diagnosis had included only the EGFR mutation assessment, without the possibility to perform the ALK rearrangement test. During the disease course, the choice to test ALK rearrangement was based on the clinical characteristics of the patient (woman, adenocarcinoma histotype, never smoker) as well as on the feasibility of a re-biopsy by minimally invasive percutaneous techniques. The case reported here confirms that the re-biopsy is an excellent tool to re-assess the disease status particularly in patients with an incomplete biomolecular baseline determination.
Furthermore, the technical accuracy of the cell blocks, obtained from fine needle aspiration cytology (FNAC), has demonstrated to be adequate both for immunohistochemistry (IHC) and molecular studies (4,10), despite the small tissular amount. In this case the reliability of this tool has led to an accurate diagnosis both for the primary lung neoplasm and its biomolecular profile and also for the second primary uterine cancer.
Again, it is highlighted the importance of achieving the biomolecular assessment also during the course of disease, and by re-biopsy if clinically indicated. This rationale relies on the actual possibility to offer a modern and targeted therapy to the patient (5). Indeed, targeted therapies in patients harboring oncogene addiction are very active and generally well tolerated, even in a personalized and proactive manner, so allowing for long-term administration. Recent reports have evidenced how in some subgroups of patients the continuation of crizotinib beyond progression could be proposed without reducing the best control of disease and leading to persisting clinical benefit (11,12).
Moreover, the integration of locoregional treatments such as surgery, radiotherapy, or other percutaneous ablative therapies, appears as a feasible multimodality strategy in selected patients with good clinical conditions and slow-growing or oligoprogressive disease (13,14). Although in absence of validated guidelines, this approach could lead to a more tailored antineoplastic therapy that exploits the multiple technical and therapeutical options in a global strategy. The favourable toxicity profile of the best known targeted agents makes proposable a combined approach; however, crizotinib is entered more recently the clinical practice and after a rapid approval due to the good results of clinical trials (7,9). Hence, the optimal therapy management and better information about some long-term or unexpected side-effects are still partially unknown, especially with regard to the safety of combined treatments.
Another interesting matter of this case report is the safe administration of crizotinib in combination with a major surgery, without complications and, importantly, with a short drug stopping. The choice of temporarily suspending crizotinib concurrently with the hysterectomy derived from a clinical precautionary judgement, in absence of specific guidelines about these clinical cases. Up to now the feasibility of integrating major surgery has not yet been well explored, as compared with stereotactic radiotherapy or ablative techniques (9,13,14). So, we think we can say that crizotinib has a good compliance also in patients requiring loco-regional and surgical treatments.
Conclusions
This case suggests that crizotinib could be safely combined with major surgery. Furthermore, this experience represents a multidisciplinary strategy both for diagnosis and treatment, therefore essential to plan more and more tailored therapies. In the future, the oncologists will tend to this objective, thanks to technological advances for molecular diagnosis applicable also to small tissues; finally, a close cooperation with the pathologists and oncologists in order to develop an optimal tissue management should be promoted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Gridelli C, Peters S, Sgambato A, et al. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev 2014;40:300-6. [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 2013;15:415-53. [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii27-39. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Chuang JC, Neal JW. Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Transl Lung Cancer Res 2015;4:639-41. [PubMed]
- Cappuzzo F, Moro-Sibilot D, Gautschi O, et al. Management of crizotinib therapy for ALK-rearranged non-small cell lung carcinoma: an expert consensus. Lung Cancer 2015;87:89-95. [PubMed]
- Stella GM, Scabini R, Inghilleri S, et al. EGFR and KRAS mutational profiling in fresh non-small cell lung cancer (NSCLC) cells. J Cancer Res Clin Oncol 2013;139:1327-35. [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Clinical impact of continued crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for ALK rearrangement. J Thorac Oncol 2013;8:654-7. [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [PubMed]
- Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys 2014;88:892-8. [PubMed]


