The role of epithelial to mesenchymal transition in resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and resistance to lung cancer therapy
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related death worldwide. The 5-year survival remains at 10–15% despite advances in treatment options. Erlotinib, a TKI targeting EGFR, was initially explored as a second-line treatment in NSCLC patients progressing on standard chemotherapy. Survival was prolonged compared to placebo (1). However, erlotinib was soon discovered to be especially efficient in patients with a mutation in the tyrosine kinase domain of EGFR. These patients are now offered EGFR-TKIs such as erlotinib and gefitinib as first-line treatment (2-4). EGFR-TKIs show an impressive response rate in EGFR-mutated patients with 70% having an objective response to treatment (5-8). Unfortunately, all patients acquire resistance over time. So far, some resistance mechanisms have been discovered including the T790M secondary mutation in EGFR, MET amplification, FGFR overexpression, IGF1R overexpression, transition to small cell lung carcinoma, gain of cancer stem-cell features, and epithelial to mesenchymal transition (EMT), but much still needs to be revealed (9-13). Up to 30% of the patients with EGFR mutations experience no objective response to EGFR-TKIs and hence appear as intrinsic resistant (14,15). Some patients without EGFR mutations initially benefit from erlotinib with experience of objective response (approx. 3%) or stable disease (approx. 25%) (3,16). Among EGFR wild-type patients, KRAS mutations have been proposed as a de novo resistance mechanism to EGFR-TKIs although the results are not unambiguous (17,18).
An urgent question is the elucidation of the molecular mechanisms behind development of TKI resistance in NSCLC since this could hold important implications for development of improved therapeutic protocols. In recent years, results assigning EMT as a candidate mechanism for mediating especially acquired EGFR-TKI resistance have emerged. Since EMT is a reversible transcriptionally regulated phenotypic shift, understanding the dynamics underlying EMT represents promising novel perspectives in therapeutic treatment of NSCLC.
Epithelial to mesenchymal transition (EMT)
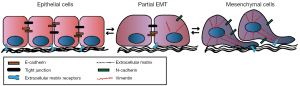
EMT is a process involved in normal embryogenesis (19). Epithelial cells appear with a distinct apical versus basolateral polarity established by dense, strong adherence and tight junctions. Epithelial cells serve as surface barriers and exercise secretory functions. During embryonic development some differentiated, polarized, epithelial cells undergo morphological changes as a response to extracellular stimulation. This process is referred to as EMT (Figure 1). The EMT process is reversible and mesenchymal-to-epithelial transformation is also a well-characterized process (19). Furthermore, EMT hold implications in cancer processes such as invasion and metastasis seen in most types of solid tumors (20). The acquirement of this invasive potential of tumor cells through EMT is interrelated with the formation of migratory, mesenchymal cells with loose cell-cell contacts and apico-basolateral polarity (21). EMT also leads to upregulation of anti-apoptotic signals rendering the cells more tumorigenic and less responsive to treatment (22,23).
EMT inducers, such as transforming growth factor-β (TGFB1) or receptor tyrosine kinase (RTK) ligands trigger changes in gene expression by activating PI3K, mitogen activated protein (MAP) kinase, RHOA and SRC signaling networks. As a consequence, transcriptional repressors like the zinc-finger proteins SNAIL (SNAI1), SLUG (SNAI2), ZEB1 and ZEB2, as well as the basic helix-loop-helix factors TWIST and E47, are upregulated (24). SNAIL, SLUG, ZEB1 and ZEB2 direct EMT by binding to E-box elements in transcriptional regulatory regions of response genes, e.g., the promoter of the gene CHD1 encoding the adherence junction-protein E-cadherin. By recruiting histone deacetylases (HDACs) and other co-repressors, the EMT mediators facilitate chromatin condensation and transcriptional repression of CHD1. E-cadherin may also be depleted through cleavage by metallo-proteases (25,26). When E-cadherin expression is reduced, adherence junctions are broken and the cells abrogate their cell polarity. This is accompanied by an increase in vimentin and N-cadherin expression (Figure 1).
E-cadherin depletion also leads to accumulation of the adherence junction component beta-catenin in the cytoplasm (27). In cooperation with Wnt-mediated signaling, beta-catenin forms a transcriptional complex with TCF/LEF and localizes to the nucleus (28). Beta-catenin/LEF here promotes gene-expression changes initiating or supporting EMT.
Complex gene-expression changes are characteristic for the EMT program and epithelial and mesenchymal cells can be distinguished by expression of a number of classical markers used for defining either epithelial or mesenchymal cells (29). Classical epithelial markers include adherence and tight-junction proteins such as E-cadherin and ZO-1, whereas the mesenchymal counterparts are frequently characterized by N-cadherin, fibronectin and vimentin (30).
EMT and treatment response to EGFR-TKIs
The EMT status has been explored as a marker in both prognosis of NSCLC and as a predictor of therapy response. It has been debated whether expression of EMT markers can predict the overall prognosis of the disease. One study performed on 95 surgically resected lung adenocarcinomas of all stages (53.7% stage I–II and 46.3% stage III–IV) found the degree of alteration of epithelial and mesenchymal markers to be significantly associated with a poor outcome (31). Another study, however, found no correlation between EMT and the disease free survival of 183 surgically resected stage I–III lung adenocarcinomas (32). The later study primarily included early stage patients (89.6% stage I–II and 10.4% stage III), which may explain the differences observed in the two studies. Another study found EMT marker status in the primary tumor to be correlated with metastatic burden (33), and vimentin expression has been shown to correlate with higher TNM status (31). Hence, EMT markers may primarily have prognostic value in later more advanced stages of NSCLC.
Since EMT is a dynamic process and tumors are prone to change from epithelial to mesenchymal in adaptation to therapy, the initial EMT status may be altered as a consequence of several rounds of treatment. The alkylating chemotherapeutic agent cisplatin, for instance, can induce EMT and confer resistance to subsequent gefitinib treatment (34). In reflection of the EMT interchangeability, Shintani et al. reported that a change towards a mesenchymal status during chemotherapy and radiation treatment was correlated with a lower disease free survival rate (35). Hence, the process and evolution of EMT in initial lines of treatment may be important for predicting the response to subsequent treatment such as EGFR-TKIs. Therefore, the inclusion of EMT status analyses during treatment could be an important clinical supplement for monitoring treatment response. Furthermore, EMT and EGFR-TKI sensitivity may be directly connected. Witta et al. reported how E-cadherin expression potentiated the sensitivity to and the apoptotic effect of gefitinib. Thus, EMT induced E-cadherin repression can directly influence and change the EGFR-TKI sensitivity (36).
Intrinsic resistance in EGFR mutated patients
The presence of EGFR mutations and an epithelial phenotype is correlated (37,38). Ren et al. found that among 99 mutated patients 57.7% appeared with epithelial or intermediary epithelial-to-mesenchymal phenotype, whereas only 25.77% was clearly mesenchymal (38). The predictive value of EMT status in EGFR mutated patients was also investigated, but no significant correlation between an epithelial phenotype and EGFR-TKI response was identified in the group of EGFR mutated patients (37,38). EGFR mutated patients in general experience a good relative response to EGFR-TKI with only 30% of patients not responding (14,15). This taken together with the tendency to an epithelial phenotype among the EGFR mutated patients suggests that the presence of an EGFR mutation overrules any influence of EMT status.
Other factors not related to mesenchymal status may explain the lack of response in some EGFR mutated patients. First, low expression of the pro-apoptotic BCL-2 family member BIM associates with an inferior response to EGFR-TKIs, but has no influence on chemotherapy response (39). Second, an explanation may also be low-level frequency of T790M mutations prior to treatment giving rise to intrinsic resistant clones (39). This is however still debated. Third, genetic polymorphisms including SNPs and differential lengths of CA-repeats within the EGFR gene can influence the response to EGFR-TKIs preferentially in EGFR mutated patients (40,41).
Treatment response in EGFR wild-type patients
Tumors from EGFR wild-type NSCLC patients constitute a biologically different group than the EGFR mutated tumors. Since EGFR mutated tumors often have an epithelial phenotype (37,38), EGFR-driven cancer may have an inherent tendency towards being epithelial. The subgroup of EGFR wild-type patients responding to EGFR-TKIs exploits another type of EGFR-dependency that may also be linked to an epithelial phenotype. In consequence, another EGFR-independent subgroup exists, which may be driven by a mechanism related to a mesenchymal phenotype. Hence, EMT status may be a valuable marker for predicting EGFR-TKI treatment response in the EGFR wild-type group. This hypothesis has been investigated and confirmed in several in vitro and in vivo studies (36-38,42-44). One example is a retrospective analysis of 99 patients with wild-type EGFR where EMT status was evaluated using a combined IHC-score for E-cadherin, N-cadherin, fibronectin and vimentin and showing that EMT status is a predictive factor for experiencing an objective response or stable disease during EGFR-TKI treatment (epithelial 23.5%, mesenchymal 2.4%) (38).
Acquired resistance in EGFR mutated patients
All initially EGFR-TKI sensitive patients will develop resistance, and alterations in cell signaling during this process may include a switch from an epithelial to a mesenchymal phenotype as one participating mechanism. However, the general lack of tumor biopsy material, representing the temporal resistance progression, limits studies of acquired resistance using clinical samples. But, some highly valuable studies have been made. Sequist et al. studied re-biopsies taken from 37 EGFR-mutated patients treated with erlotinib or gefitinib (13). For this study biopsies were taken at the time of progression according to the RECIST criteria. Among the 37 re-biopsies they observed transition to SCLC (5), MET amplification (3), EGFR T790M mutations (21), EGFR amplification (4) and EMT (3). Some cases showed heterogeneity with co-occurrence of T790M and other alterations such as EGFR amplification. In seven cases, no genetic alteration was found, and in 3 of these cases EMT was detected. Evaluation of EMT was only performed in the seven re-biopsies without known resistance mechanism and in 5 of the re-biopsies presenting with EGFR T790M mutations (13). Similarly, EMT was not investigated in a large re-biopsy study performed on 155 patients (45).
Since only a few re-biopsies have been evaluated for EMT, it is difficult to estimate a quantitative measure of the general occurrence of EMT alone or accompanying other resistance mechanisms. Thus, the occurrence of EMT could in principle be much higher than reported so far. Notably, EMT were reported to co-occur with EGFR T790M mutations in another study using re-biopsies (46), and was also evident in a metastatic lesion at time of progression in a Korean case study (47). As a contrast to the sparse investigation of EMT as an acquired resistance mechanism in EGFR-TKI treated patients, numerous cell studies exploring and highlighting the importance of EMT in TKI resistance development exists as elaborated below.
Molecular mechanisms for EMT induction related to EGFR-TKI resistance
EMT can be induced and regulated by various growth and differentiation pathways and factors. These include Wnt and Notch proteins, NFκB pathway, TGF-β, and RTKs and activating growth factors: IGF1, GAS6, HGF, FGF and EGF. Several links between EMT-induction and EGFR-TKI treatment exists (Figure 2). In the instance of Notch-induced EMT, Notch is activated during EGFR-TKI treatment as EGFR signaling normally has a repressive effect on this pathway (48).

Moreover, EGFR-TKI treatment can create a selective pressure favoring cells capable of bypass signaling through activating of RTKs or TGFbR and subsequent activation of SMAD, PI3K/AKT and MAP kinase pathways. Co-occurring induction of EMT may result in generation of mesenchymal-like cells which have a growth and survival advantage do to the lack of E-cadherin expression and through the anti-apoptotic effect of the EMT factors SNAIL, SLUG and ZEB1 (22,23,49).
TGF-β
Cancer cells can increase their production of active TGF-β during development of EGFR-TKI resistance. TGF-β triggers EMT and allow the cells to become invasive (50) (Figure 2). Furthermore, transient TGF-β-stimulation induces EMT and acquired resistance to EGFR-TKIs in NSCLC cell lines (51-53). TGF-β is the most studied inducer of EMT and initiates the process primarily through activation of the SMAD-pathway. Upon ligand-binding to the TGF-β receptor complex, SMAD2 and SMAD3 are activated through phosphorylation by TGFbRI (54). SMAD4, consecutively, forms a trimer with SMAD2 and SMAD3 and translocates into the nucleus. Through interaction with other DNA-binding transcription factors, SMAD4 represses transcription of epithelial genes such as CDH1 or activates transcription of mesenchymal genes (55). Several downstream transcription factors including SNAIL, SLUG, ZEB1, ZEB2 and basic helix-loop-helix family members are activated and mediate further downstream effects (50). In addition, TGF-β may also activate the PI3K/AKT and MAP kinase pathways. Although TGFbR is a serine-threonine kinase, a few tyrosine residues are present. When these are phosphorylated, they create a docking site for Src homology-2-domain containing proteins, like PI3K and GRB2, creating a link to the PI3K/AKT and MAP kinase pathways (56). PI3K/AKT and MAPK activation by TGF-β can further be potentiated by simultaneous stimulation with additional growth factors (54,57).
Receptor tyrosine kinases (RTKs)
RTKs predominantly signal through the PI3K/AKT and MAP kinase pathways. Activation of the PI3K/AKT cell survival pathway protects against apoptosis while driving pathways critical to carcinogenesis (58). MAP kinase, or, signaling primarily stimulates mitosis and hence cell proliferation (59). In addition, RTKs, such as MET, IGF-1R, FGFRs and the non-RTK c-Src have been reported to induce phosphorylation of E-cadherin and associated catenins, resulting in their degradation (60). Bypass-signaling through activation of a distinct RTK is often seen during acquired EGFR-TKI resistance (61). Although EMT can be induced by RTKs, the process may later be independent of RTK signaling (62). Different stages of EMT exist, including a partial EMT stage with cell contacts, but upregulation of mesenchymal markers. During treatment with EGFR-TKIs EMT may be initiated in the tumor cells, resembling a partial EMT. This could be caused by increased expression of TGF-β (52). Surprisingly, drug-withdrawal may further enhance the EMT state due to an EGFR-mediated boost of a partial EMT existing in a “ready-to-go” fashion (63). A recent study suggested a role for TGF-β in mediating the RTK switch with accompanying activation of AKT and EMT, hence, rendering cells independent of EGFR signaling (52). An increase in TGF-β during resistance development could create an autocrine loop, linking the kinase switch and EMT. Due to the lack of studies on biopsies from patients developing EGFR-TKI resistance, there is limited data on the co-occurrence of kinase switch and EMT in vivo. Moreover, due to limited biopsy material, EMT has in most cases only been studied when no other resistance mechanism appeared (13). Below is the relation between EMT in lung cancer and the RTKs IGF1R, AXL, and MET specifically highlighted.
IGF1R
From in vitro experiments, it is known that EMT can be initiated by stimulation with IGF1 and reversed when adding the IGF1R inhibitor AEW541 (64). IGF1R activation is also a proposed resistance mechanism to EGFR TKIs in NSCLC cell lines (53,65). In PC9 NSCLC cells made erlotinib-resistant in vitro, EMT was reversed upon IGF1R inhibition with I-OMe-AG538 indicating a role for IGF1R in TKI resistance related EMT (53). A study on IGF1R overexpressing cells highlighted the EMT transcription factor SNAIL as the downstream effector of IGF1R-induced EMT (66).
Our own studies (unpublished results) and studies from others suggest a role for IGF1R in EMT initiation, but also that established mesenchymal-like lung adenocarcinoma cells no longer depended on IGF1R signaling for proliferation or survival (67). This indicates that IGF1R signaling can induce EMT, but once these cells have transitioned to a mesenchymal state they are not necessarily reliant on IGF1R signaling (Figure 2).
AXL
AXL belongs to the TYRO3, AXL and MERTK (TAM) family of RTKs characterized by having two immunoglobulin-like domains as well as fibronectin repeat domains in the extracellular region. AXL and the ligand GAS6 are reported as overexpressed in cancer (68). Upregulation of the RTK AXL has also been implicated in acquired erlotinib resistance in vivo and in vitro, and AXL inhibition comprises a promising target for restoring erlotinib sensitivity (42,69). In a HCC827-derived model, erlotinib-resistant cell clones upregulated AXL with subsequent EMT (69). AXL activation restored AKT, MAPK and Notch signaling while inhibition with RNAi or molecular inhibitors restored erlotinib sensitivity and decreased signaling (69). The knockdown of vimentin likewise restored erlotinib sensitivity and decreased AXL expression, and since no changes were seen in vimentin levels upon AXL inhibition, the state of EMT have become independent of AXL signaling (69). Alternatively AXL upregulation may be an event downstream of EMT.
MET
MET, also called c-met and hepatocyte growth factor receptor (HGFR), has HGF as the only known ligand. MET is normally expressed by cells of epithelial origin, while expression of HGF is restricted to cells of mesenchymal origin (70). HGF mediated EMT induces resistance to chemotherapy in SCLC (71), but so far no reports on MET induced EMT and resistance to EGFR-TKIs in NSCLC exist. Upon HGF stimulation, MET induces several biological responses related to invasive cell growth and abnormal MET activation in cancer correlates with poor prognosis. HGF and MET dependent activation of the MAPK pathway leads to c-FOS mediated upregulation of EGR-1 transcription. EGR-1 is a positive transcription factor for SNAIL, implicating SNAIL upregulation as a core event in HGF-induced EMT (72). MET gene amplification is one of the most frequently reported resistance mechanisms in NSCLC, and it will be important to identify if EMT contributes to the MET-amplification-mediated resistant phenotype or if resistance mechanisms are mutually exclusive.
Drug mediated repression of EMT in NSCLC
In addition to the studies addressing EMT as a potential resistance mechanism in NSCLC, several studies have also addressed the potential therapeutic targeting of EMT. Two such EMT targeting drug-types under investigation are HDAC inhibitors [e.g., entinostat (MS-275)] and MEK-inhibitors (e.g., selumetinib) (Figure 3). Entinostat abolishes the deacetylation of histones and accordingly helps keeping chromatin in an active, transcribed form. As E-cadherin transcription is regulated through epigenetics, HDAC-inhibitors may induce increased E-cadherin transcription and mesenchymal to epithelial transition (73). In addition, entinostat increases the effect of erlotinib in resistant cell lines (36). This finding led to a phase II clinical trial for entinostat and erlotinib co-treatment. No difference in progression-free survival (PFS), overall survival, or objective response was observed by combining entinostat with erlotinib for the entire group of patients (74). There was, however, a prolonged PFS for patients expressing high levels of E-cadherin at the beginning of the trial. This could indicate that entinostat delays the onset of acquired resistance to erlotinib by preventing the establishment of resistant EMT subclones by keeping the cells in an epithelial state and calls for further biomarker-driven studies addressing this (74).
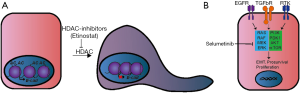
Pre-treatment of cells with the MEK-inhibitor selumitinib reverses EMT and sensitize cells to EGFR inhibitors (75). Interestingly, selumetinib was effective in inhibiting TKI-resistant Calu-3 derived NSCLC cells as well as the parental Calu-3 cells, emphasizing the central role of MEK in intracellular signaling (67). A recent clinical phase 2 trial of selumetinib together with the anti-mitotic chemotherapeutic docetaxel showed improved PFS for KRAS mutated stage IIIb–VI lung cancer patients (76). Inhibition of MEK with targeted drugs like selumetinib hereby shows promising potential in patients with inferior EGFR-TKI response rates such as patients with KRAS mutations or with acquired resistance including EMT related cases.
Conclusions
EMT appears an important event in development of TKI resistance in NSCLC cells, and numerous studies show how EMT influences the EGFR-TKI treatment response. Both intrinsic and acquired resistance to EGFR-TKIs can be influenced by the EMT-status of the tumor and this accordingly represents an important factor both for prognosis and selection of treatment protocol.
Acquired resistance to EGFR-TKIs has proven to be a highly circuitous event. Multiple mechanisms exist, and heterogeneity resulting in co-occurrence of different resistance mechanisms is far from unusual. EMT is one such mechanism to be explored both as an independent mechanism as well in the context of other resistance mechanisms. Due to the reversibility of EMT, pharmaceutical targeting of EMT provides hope for counteracting both intrinsic and acquired NSCLC resistance resulting in a higher response rate for EGFR-TKIs and accordingly superior patient survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in Previously Treated Non-Small-Cell Lung Cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Sequist LV, Joshi VA, Jänne PA, et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist 2007;12:90-8. [Crossref] [PubMed]
- Weber B, Hager H, Sorensen BS, et al. EGFR mutation frequency and effectiveness of erlotinib: a prospective observational study in Danish patients with non-small cell lung cancer. Lung Cancer 2014;83:224-30. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Tenen DG, et al. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer 2007;58:95-103. [Crossref] [PubMed]
- Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res 2009;15:5267-73. [Crossref] [PubMed]
- Miller VA, Riely GJ, Zakowski MF, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol 2008;26:1472-8. [Crossref] [PubMed]
- Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. [Crossref] [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [Crossref] [PubMed]
- Ware KE, Hinz TK, Kleczko E, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis 2013;2:e39. [Crossref] [PubMed]
- Shien K, Toyooka S, Yamamoto H, et al. Acquired Resistance to EGFR Inhibitors Is Associated with a Manifestation of Stem Cell-like Properties in Cancer Cells. Cancer Res 2013;73:3051-61. [Crossref] [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Wang Y, Schmid-Bindert G, Zhou C. Erlotinib in the treatment of advanced non-small cell lung cancer: an update for clinicians. Ther Adv Med Oncol 2012;4:19-29. [Crossref] [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [Crossref] [PubMed]
- Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008;9:962-72. [Crossref] [PubMed]
- Ferté C, Besse B, Dansin E, et al. Durable responses to Erlotinib despite KRAS mutations in two patients with metastatic lung adenocarcinoma. Ann Oncol 2010;21:1385-7. [Crossref] [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740-6. [Crossref] [PubMed]
- Thiery JP. Epithelial - mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54. [Crossref] [PubMed]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 2010;15:117-34. [Crossref] [PubMed]
- Vega S, Morales AV, Ocaña OH, et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 2004;18:1131-43. [Crossref] [PubMed]
- Takeyama Y, Sato M, Horio M, et al. Knockdown of ZEB1, a master epithelial-to-mesenchymal transition (EMT) gene, suppresses anchorage-independent cell growth of lung cancer cells. Cancer Lett 2010;296:216-24. [Crossref] [PubMed]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007;7:415-28. [Crossref] [PubMed]
- Cowden Dahl KD, Symowicz J, Ning Y, et al. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res 2008;68:4606-13. [Crossref] [PubMed]
- Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2010;2:a002915. [Crossref] [PubMed]
- Orsulic S, Huber O, Aberle H, et al. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci 1999;112:1237-45. [PubMed]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741-51. [Crossref] [PubMed]
- LaGamba D, Nawshad A, Hay ED. Microarray analysis of gene expression during epithelial-mesenchymal transformation. Dev Dyn 2005;234:132-42. [Crossref] [PubMed]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009;119:1429-37. [Crossref] [PubMed]
- Shi Y, Wu H, Zhang M, et al. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol 2013;8:89. [Crossref] [PubMed]
- Chikaishi Y, Uramoto H, Tanaka F. The EMT status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res 2011;31:4451-6. [PubMed]
- Huang J, Qiu Y, Chen G, et al. The relationship between Bmi-1 and the epithelial-mesenchymal transition in lung squamous cell carcinoma. Med Oncol 2012;29:1606-13. [Crossref] [PubMed]
- Kurokawa M, Ise N, Omi K, et al. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci 2013;104:904-11. [Crossref] [PubMed]
- Shintani Y, Okimura A, Sato K, et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg 2011;92:1794-804; discussion 1804.
- Witta SE, Gemmill RM, Hirsch FR, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res 2006;66:944-50. [Crossref] [PubMed]
- Chen B, Xiao F, Li B, et al. The role of epithelial-mesenchymal transition and IGF-1R expression in prediction of gefitinib activity as the second-line treatment for advanced nonsmall-cell lung cancer. Cancer Invest 2013;31:454-60. [Crossref] [PubMed]
- Ren S, Su C, Wang Z, et al. Epithelial phenotype as a predictive marker for response to EGFR-TKIs in non-small cell lung cancer patients with wild-type EGFR. Int J Cancer 2014;135:2962-71. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [Crossref] [PubMed]
- Winther-Larsen A, Nissen PH, Jakobsen KR, et al. Genetic polymorphism in the epidermal growth factor receptor gene predicts outcome in advanced non-small cell lung cancer patients treated with erlotinib. Lung Cancer 2015;90:314-20. [Crossref] [PubMed]
- Winther Larsen A, Nissen PH, Meldgaard P, et al. EGFR CA repeat polymorphism predict clinical outcome in EGFR mutation positive NSCLC patients treated with erlotinib. Lung Cancer 2014;85:435-41. [Crossref] [PubMed]
- Byers LA, Diao L, Wang J, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res 2013;19:279-90. [Crossref] [PubMed]
- Yauch RL, Januario T, Eberhard DA, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res 2005;11:8686-98. [Crossref] [PubMed]
- Frederick BA, Helfrich BA, Coldren CD, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther 2007;6:1683-91. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Uramoto H, Shimokawa H, Hanagiri T, et al. Expression of selected gene for acquired drug resistance to EGFR-TKI in lung adenocarcinoma. Lung Cancer 2011;73:361-5. [Crossref] [PubMed]
- Chung JH, Rho JK, Xu X, et al. Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer 2011;73:176-82. [Crossref] [PubMed]
- Arasada RR, Amann JM, Rahman MA, et al. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res 2014;74:5572-84. [Crossref] [PubMed]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009;27:2059-68. [Crossref] [PubMed]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 2009;19:156-72. [Crossref] [PubMed]
- Kitamura K, Seike M, Okano T, et al. MiR-134/487b/655 Cluster Regulates TGF- -Induced Epithelial-Mesenchymal Transition and Drug Resistance to Gefitinib by Targeting MAGI2 in Lung Adenocarcinoma Cells. Mol Cancer Ther 2014;13:444-53. [Crossref] [PubMed]
- Izumchenko E, Chang X, Michailidi C, et al. The TGFβ-miR200-MIG6 pathway orchestrates the EMT-associated kinase switch that induces resistance to EGFR inhibitors. Cancer Res 2014;74:3995-4005. [Crossref] [PubMed]
- Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, et al. IGF-1R/epithelial-to-mesenchymal transition (EMT) crosstalk suppresses the erlotinib-sensitizing effect of EGFR exon 19 deletion mutations. Sci Rep 2013;3:2560. [Crossref] [PubMed]
- Grände M. Transforming growth factor-beta and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci 2002;115:4227-36. [Crossref] [PubMed]
- Miyaki M, Kuroki T. Role of Smad4 (DPC4) inactivation in human cancer. Biochem Biophys Res Commun 2003;306:799-804. [Crossref] [PubMed]
- Huang F, Chen YG. Regulation of TGF-β receptor activity. Cell Biosci 2012;2:9. [Crossref] [PubMed]
- Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res 2009;19:128-39. [Crossref] [PubMed]
- Barr S, Thomson S, Buck E, et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis 2008;25:685-93. [Crossref] [PubMed]
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004;23:2838-49. [Crossref] [PubMed]
- Fujita Y, Krause G, Scheffner M, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 2002;4:222-31. [Crossref] [PubMed]
- Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol 2013;31:3987-96. [Crossref] [PubMed]
- Thomson S, Petti F, Sujka-Kwok I, et al. A systems view of epithelial-mesenchymal transition signaling states. Clin Exp Metastasis 2011;28:137-55. [Crossref] [PubMed]
- La Monica S, Caffarra C, Saccani F, et al. Gefitinib inhibits invasive phenotype and epithelial-mesenchymal transition in drug-resistant NSCLC cells with MET amplification. PLoS One 2013;8:e78656. [Crossref] [PubMed]
- Nurwidya F, Takahashi F, Kobayashi I, et al. Treatment with insulin-like growth factor 1 receptor inhibitor reverses hypoxia-induced epithelial-mesenchymal transition in non-small cell lung cancer. Biochem Biophys Res Commun 2014;455:332-8. [Crossref] [PubMed]
- Suda K, Mizuuchi H, Sato K, et al. The insulin-like growth factor 1 receptor causes acquired resistance to erlotinib in lung cancer cells with the wild-type epidermal growth factor receptor. Int J Cancer 2014;135:1002-6. [Crossref] [PubMed]
- Kim HJ, Litzenburger BC, Cui X, et al. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol 2007;27:3165-75. [Crossref] [PubMed]
- Morgillo F, Cascone T, D’Aiuto E, et al. Antitumour efficacy of MEK inhibitors in human lung cancer cells and their derivatives with acquired resistance to different tyrosine kinase inhibitors. Br J Cancer 2011;105:382-92. [Crossref] [PubMed]
- Wu X, Liu X, Koul S, et al. AXL kinase as a novel target for cancer therapy. Oncotarget 2014;5:9546-63. [Crossref] [PubMed]
- Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852-60. [Crossref] [PubMed]
- Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011;3:S7-19. [Crossref] [PubMed]
- Cañadas I, Rojo F, Taus Á, et al. Targeting epithelial-to-mesenchymal transition with Met inhibitors reverts chemoresistance in small cell lung cancer. Clin Cancer Res 2014;20:938-50. [Crossref] [PubMed]
- Grotegut S, von Schweinitz D, Christofori G, et al. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J 2006;25:3534-45. [Crossref] [PubMed]
- Suda K, Tomizawa K, Fujii M, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol 2011;6:1152-61. [Crossref] [PubMed]
- Witta SE, Jotte RM, Konduri K, et al. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol 2012;30:2248-55. [Crossref] [PubMed]
- Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res 2014;74:309-19. [Crossref] [PubMed]
- Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38-47. [Crossref] [PubMed]


