A potential role for estrogen in cigarette smoke-induced microRNA alterations and lung cancer
Introduction
Lung cancer (LC) is the first and foremost cause of cancer death (1). LC is histologically classified in small cell lung cancer (SCLC), which is a neuroendocrine tumor, and non-small cell lung cancer (NSCLC), which arises from epithelial cells of the airways (2,3), and represents 80% of cases (1), including major subtypes such as lung adenocarcinoma (ADC), squamous cell carcinoma (SCC), and large cell carcinoma (LCC) (2,3). Currently, the trend in incidence rates of LC in women is slightly higher compared to men, and such differences could be due to an increased susceptibility to the carcinogenic effects of cigarette-smoke (CS) in women (4), and a consequence of the historical differences in tobacco use (1).
Sex steroids modulate airway and lung functioning, mainly estrogens via estrogen receptors (ERα or ERβ) (5-7), and their alterations by CS can be associated with any type of lung disease (8-11). Some reports suggest the development of NSCLC is correlated between the expression of ER (α or β) with aromatase expression and/or the epidermal growth factor receptor (EGFR) pathway (12,13). The major histologic subtype of LC in women is the ADC, which can be related to ER expression by endogenous or exogenous estrogens (4,14,15). In non-smoking women the development of some LC has been associated to mutation of EGFR by ERα expression (16), although there is also information that androgen receptor (AR) and EGFR crosstalk contributes to LC progression in men (17). ERs, and other nuclear receptors, can regulate directly or indirectly microRNAs (miRNAs), suggesting that control of miRNA expression can happen at both the transcriptional and maturation levels (18). These post-transcriptional gene regulators control many known oncogenes, and can also act on their own as either oncogenes or tumor suppressors (19). MiRNA up-regulation has been associated with genomic amplification (20,21), and miRNA down-regulation has been associated with chromosomal deletions, point mutations and aberrant promoter methylation (22-24). Many miRNAs are usually down-regulated in human LC tissues while others are up-regulated (Table 1), and seem to work differently depending on the cellular context (47). However, developing any type of LC by deregulation of miRNAs via ER-Estrogen effect is still not entirely clear (8).
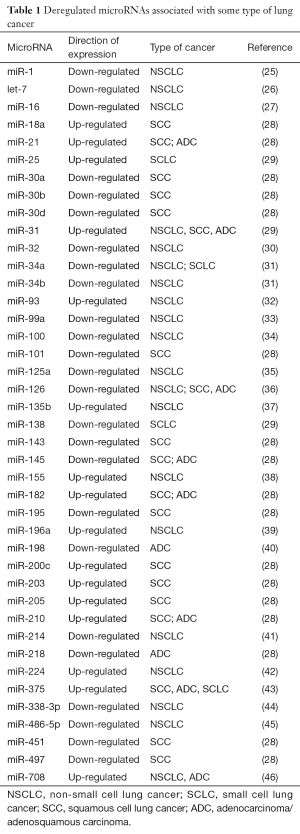
Full table
miRNAs repression by cigarette smoke in the lungs
We have summarized before the results showing reduced expression levels of miRNAs in various tumors and cancer cell lines, including LC (48). The observation of a global miRNA repression in the lungs of rodents exposed to CS has also been reported (49-52). Izzotti et al. show in their results the extensive down-regulation of 126 miRNAs in lungs of rats, 4 weeks after CS exposure (49). Such a short-lasting exposure to CS resulted in reversible miRNA alterations, as miRNA down-regulation was considerably attenuated one week after smoking cessation (51). By contrast, the repression of miRNA detected in mice exposed to CS for 4 months still persisted 3 months after smoking cessation, with the progressive development of cancer in the lung, suggesting that long-lasting exposure is needed to induce irreversible miRNA alterations (51,53). In their previous published results, using the same animal model, Izzotti et al. have found that CS up-regulates gene transcription and protein expression (54,55). The authors suggested that the CS-induced down-regulation of miRNA cause oncogene activation and cell proliferation, and that the persistence of these molecular alterations is crucial to commit lung cells towards carcinogenesis (51,53). These results are supported by the study of Schembri et al., who found that most of the differentially expressed miRNAs in the human bronchial airway epithelium were down-regulated in smokers and were inversely correlated with their predicted targets (56). The same phenomenon was also observed in alveolar macrophages of smokers, where CS decreased global miRNA expression, while increased their predicted targets (57). In this later study the decrease in global miRNA expression was more pronounced in heavy smokers, suggesting that the magnitude of miRNA repression is related to the extent of smoking history (57).
Anti-estrogenic compounds as attenuators of cigarette smoke effects
Studies suggest that phytochemicals from vegetables and fruits exhibit chemopreventive activities against various types of cancer (58). Izzotti et al. (59) evaluated miRNA expression in the lungs of rats exposed to CS and treated with several cancer chemopreventive agents. Administration of the dietary agents, Phenethyl isothiocyanate (PEITC) and Indole-3-carbinol (I3C), two major components of cruciferous vegetables, attenuated the cigarette smoke-induced down-regulation of miRNA expression. In the case of the combined treatment with PEITC and I3C, they had profound effects on almost all CS—down-regulated miRNAs and their expression even exceeded the baseline situation (59). In previous studies, PEITC was also the most effective agent in inhibition of CS-related cytogenetic damage, transcriptome alterations, and lung tumorigenesis (55,60,61). Also, I3C and its condensation product 3,3’-diindolylmethane (DIM) exhibit potent antitumor effects with negligible levels of toxicity in a wide range of human cancer cells, including LC (62). Interestingly, both PEITC and I3C have proved to be anti-estrogenic compounds and inhibited ERα expression (63-66).
A similar effect was seen when mice were treated with the anti-diabetic drug Metformin (67). Exposure of mice to CS resulted in down-regulation of miRNA expression in the lungs after 10 weeks. Preneoplastic lesions in the lung were detectable after 7.5 months, along with lung tumors. Metformin effectively changed the miRNA alterations resulted from exposure to CS in the mouse lung and normalized the expression of several down-regulated miRNAs. It did not prevent smoke-induced lung tumors, however, it inhibited preneoplastic lesions in the lung (67). Intriguingly, there is evidence that Metformin is also an anti-estrogenic molecule. It was shown to inhibit ERα expression in cancer cells and to decrease estrogen levels in the serum of breast cancer female patients (68,69).
Sex differences in LC susceptibility
Estrogens seem to play a main role in development and progression of LC in women (70,71). ERα and ERβ are frequently expressed in lung tissue, lung tumors and LC cell lines (72), and may accelerate the metabolism of smoke related carcinogens in a dose-dependent way, as suggested by higher levels of polycyclic aromatic hydrocarbons-DNA adducts in female smokers (70).
Intergender differences were revealed in lung tumor multiplicities and in pulmonary miRNA expression after exposure of mice to CS (73,74). Females were found to be more susceptible to CS-induced miRNA down-regulation, while on the other hand; a more remarkable induced-protein expression by CS was detected in females than in males (74). These results raise the possibility that the hormone estrogen might regulate the CS-induced miRNA alterations in the lungs. Estrogens and their receptors were detected within murine lung tissue (75,76), and there is evidence for higher susceptibility of women to smoking-related LC (77-79), and that anti-estrogens can prevent lung tumorigenesis (80). Moreover, CS was found to accelerate the production of the carcinogenic estrogenic metabolites 4-OHEs in the lungs (76), and the role that estrogen-metabolizing enzymes such as Cytochrome P450 1b1 (CYP1B1) may have in the enhanced female gender-related susceptibility to tobacco has also been previously reported (75,81,82). The CS carcinogen nicotine-derived nitrosamine ketone (NNK) was shown to induce ERα via CYP1B1 activation (83), and anti-estrogens effectively inhibited NNK-induced murine lung carcinogenesis (84).
The potential role of estrogen in cigarette smoke—related miRNAs alterations
We have previously shown that estrogens cause widespread down-regulation of miRNAs, similarly to their reduced miRNA expression levels observed in different tumors and cancer cell lines (48,85). Herein we suggest that elevated levels of estrogens or their metabolites inside the lungs as a result of CS exposure [or xenoestrogens (48) within CS] cause the observed CS-induced down-regulation of miRNAs (Table 2) that potentially can lead to up-regulation of their target proto-oncogenes, cellular proliferation and lung tumor development. These alterations by estrogen can occur as the result of affecting the transcription of miRNA genes, as was shown in the case of global miRNA regulation by c-Myc (87), or at any stage of the miRNA maturation process, whether at the level of pri-miRNA processing, by affecting the microprocessor complex Drosha-DGCR8, or at the pre-miRNA processing level, by altering the activity of Dicer (Figure 1). Deletion of Dicer revokes the production of mature miRNAs being a conditional for lung tumor development as observed in a mouse model (89). Of note, it was suggested that Dicer, which was found to be inhibited by multiple stressors, is a main component of the stress-response machinery that triggers the miRNA response to carcinogens (90,91), as was observed in alveolar macrophages, where cigarette smoke-related changes in Dicer decreased miRNA expression (92).
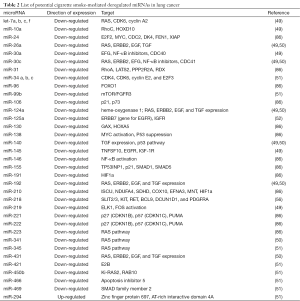
Full table
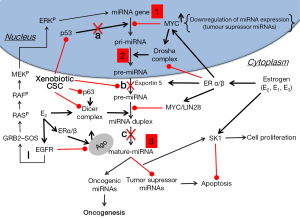
It is worth mentioning here that EGFR was shown to suppress the maturation of tumor-suppressor-like miRNAs in response to hypoxic stress through phosphorylation of Argonaute 2 (AGO2), which in turn reduces the binding of Dicer to AGO2 and inhibits miRNA processing from precursor miRNAs to mature miRNAs (93). Rapid non-genomic estrogenic pathways are mediated by cell membrane receptors such as EGFR, and because of this crosstalk the effectiveness of EGFR tyrosine kinase inhibitors can be enhanced by anti-estrogens and aromatase inhibitors, showing antitumor effects in LC (80). However, EGFR can also provide alternative proliferation and survival stimuli to the tumors in the presence of effective inhibition of the ER pathway and therefore it has a major role in acquired endocrine therapy resistance (94).
Perspectives
Elucidating the molecular mechanisms of miRNA repression after exposure to CS, the target genes of the CS-down-regulated miRNAs and the potential involvement of estrogen in these alterations can have clinical implications for prevention and treatment of LC. The use of anti-estrogenic compounds, such as those found in cruciferous vegetables, can help attenuate these effects and potentially reduce the risk of LC among smokers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. Ca Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Heighway J, Betticher DC. Lung tumors: an overview. Atlas Genet Cytogenet Oncol Haematol 2004;8:139-41.
- Watanabe T, Miura T, Degawa Y, et al. Comparison of lung cancer cell lines representing four histopathological subtypes with gene expression profiling using quantitative real-time PCR. Cancer Cell Int 2010;10:2. [Crossref] [PubMed]
- Fu JB, Kau TY, Severson RK, et al. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest 2005;127:768-77. [Crossref] [PubMed]
- Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L866-70. [Crossref] [PubMed]
- Townsend EA, Thompson MA, Pabelick CM, et al. Rapid effects of estrogen on intracellular ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2010;298:L521-30. [Crossref] [PubMed]
- Carey MA, Card JW, Voltz JW, et al. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 2007;293:L272-8. [Crossref] [PubMed]
- Verma MK, Miki Y, Sasano H. Sex steroid receptors in human lung diseases. J Steroid Biochem Mol Biol 2011;127:216-22. [Crossref] [PubMed]
- Brand JS, Chan MF, Dowsett M, et al. Cigarette smoking and endogenous sex hormones in postmenopausal women. J Clin Endocrinol Metab 2011;96:3184-92. [Crossref] [PubMed]
- Sathish V, Martin YN, Prakash YS. Sex steroid signaling: Implications for lung diseases. Pharmacol Ther 2015;150:94-108. [Crossref] [PubMed]
- Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders Eur J Endocrinol 2005;152:491-9. [Crossref] [PubMed]
- Shen L, Li Z, Shen S, et al. The synergistic effect of EGFR tyrosine kinase inhibitor gefitinib in combination with aromatase inhibitor anastrozole in non-small cell lung cancer cell lines. Lung Cancer 2012;78:193-200. [Crossref] [PubMed]
- Verma MK, Miki Y, Sasano H. Aromatase in human lung carcinoma. Steroids 2011;76:759-64. [Crossref] [PubMed]
- Di Nunno L, Larsson LG, Rinehart JJ, et al. Estrogen and progesterone receptors in nonsmall cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med 2000;124:1467-70. [PubMed]
- Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of alpha and beta estrogen receptors in human nontumor and tumor lung tissue. Mol Cell Endocrinol 2002;188:125-40. [Crossref] [PubMed]
- Raso MG, Behrens C, Herynk MH, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res 2009;15:5359-68. [Crossref] [PubMed]
- Recchia AG, Musti AM, Lanzino M, et al. A cross-talk between the androgen receptor and the epidermal growth factor receptor leads to p38MAPK-dependent activation of mTOR and cyclinD1 expression in prostate and lung cancer cells. Int J Biochem Cell Biol 2009;41:603-14. [Crossref] [PubMed]
- Yang Z, Wang L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci 2011;1:31. [Crossref] [PubMed]
- Ferracin M, Calin GA, Negrini M. MicroRNAs in cancer (an overview). In: Cho WC, editor. MicroRNAs in cancer translational research. 1st ed. Dordrecht: Springer Netherlands, 2011:1-71.
- He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature 2005;435:828-33. [Crossref] [PubMed]
- O’Donnell KA, Wentzel EA, Zeller KI, et al. C-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005;435:839-43. [Crossref] [PubMed]
- Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002;99:15524-9. [Crossref] [PubMed]
- Datta J, Kutay H, Nasser MW, et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res 2008;68:5049-58. [Crossref] [PubMed]
- Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006;9:435-43. [Crossref] [PubMed]
- Zhao Q, Zhang B, Shao Y, et al. Correlation between the expression levels of miR-1 and PIK3CA in non-small-cell lung cancer and their relationship with clinical characteristics and prognosis. Future Oncol 2014;10:49-57. [Crossref] [PubMed]
- Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell 2005;120:635-47. [Crossref] [PubMed]
- Ke Y, Zhao W, Xiong J, et al. Downregulation of miR-16 promotes growth and motility by targeting HDGF in non-small cell lung cancer cells. FEBS Lett 2013;587:3153-7. [Crossref] [PubMed]
- Guan P, Yin Z, Li X, et al. Meta-analysis of human lung cancer microRNA expression profiling studies comparing cancer tissues with normal tissues. J Exp Clin Cancer Res 2012;31:54. [Crossref] [PubMed]
- Naidu S, Garofalo M. microRNAs: An Emerging Paradigm in Lung Cancer Chemoresistance. Front Med (Lausanne) 2015;2:77. [PubMed]
- Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006;9:189-98. [Crossref] [PubMed]
- Gallardo E, Navarro A, Viñolas N, et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis 2009;30:1903-9. [Crossref] [PubMed]
- Zhu W, He J, Chen D, et al. Expression of miR-29c, miR-93, and miR-429 as potential biomarkers for detection of early stage non-small lung cancer. PLoS One 2014;9:e87780. [Crossref] [PubMed]
- Chen C, Zhao Z, Liu Y, et al. microRNA-99a is downregulated and promotes proliferation, migration and invasion in non-small cell lung cancer A549 and H1299 cells. Oncol Lett 2015;9:1128-34. [PubMed]
- Liu J, Lu KH, Liu ZL, et al. MicroRNA-100 is a potential molecular marker of non-small cell lung cancer and functions as a tumor suppressor by targeting polo-like kinase 1. BMC Cancer 2012;12:519. [Crossref] [PubMed]
- Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 2004;101:2999-3004. [Crossref] [PubMed]
- Sun Y, Bai Y, Zhang F, et al. miR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem Biophys Res Commun 2010;391:1483-9. [Crossref] [PubMed]
- Lin CW, Chang YL, Chang YC, et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun 2013;4:1877. [Crossref] [PubMed]
- Donnem T, Lonvik K, Eklo K, et al. Independent and tissue-specific prognostic impact of miR-126 in nonsmall cell lung cancer: coexpression with vascular endothelial growth factor-A predicts poor survival. Cancer 2011;117:3193-200. [Crossref] [PubMed]
- Liu XH, Lu KH, Wang KM, et al. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer 2012;12:348. [Crossref] [PubMed]
- Wu S, Zhang G, Li P, et al. miR-198 targets SHMT1 to inhibit cell proliferation and enhance cell apoptosis in lung adenocarcinoma. Tumour Biol 2016;37:5193-202. [Crossref] [PubMed]
- Salim H, Arvanitis A, de Petris L, et al. miRNA-214 is related to invasiveness of human non-small cell lung cancer and directly regulates alpha protein kinase 2 expression. Genes Chromosomes Cancer 2013;52:895-911. [Crossref] [PubMed]
- Cui R, Meng W, Sun HL, et al. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci U S A 2015;112:E4288-97. [Crossref] [PubMed]
- Jin Y, Liu Y, Zhang J, et al. The expression of miR-375 is associated with carcinogenesis in three subtypes of lung cancer. PLoS One 2015;10:e0144187. [Crossref] [PubMed]
- Sun J, Feng X, Gao S, et al. microRNA-338-3p functions as a tumor suppressor in human non-small-cell lung carcinoma and targets Ras-related protein 14. Mol Med Rep 2015;11:1400-6. [PubMed]
- Wang J, Tian X, Han R, et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 2014;33:1181-9. [Crossref] [PubMed]
- Jang JS, Jeon HS, Sun Z, et al. Increased miR-708 expression in NSCLC and its association with poor survival in lung adenocarcinoma from never smokers. Clin Cancer Res 2012;18:3658-67. [Crossref] [PubMed]
- Guz M, Rivero-Müller A, Okoń E, et al. MicroRNAs-role in lung cancer. Dis Markers 2014;2014:218169.
- Cohen A, Burgos-Aceves MA, Smith Y. Estrogen repression of microRNA as a potential cause of cancer. Biomed Pharmacother 2016;78:234-8. [Crossref] [PubMed]
- Izzotti A, Calin GA, Arrigo P, et al. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 2009;23:806-12. [Crossref] [PubMed]
- Izzotti A, Calin GA, Steele VE, et al. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J 2009;23:3243-50. [Crossref] [PubMed]
- Izzotti A, Larghero P, Longobardi M, et al. Dose-responsiveness and persistence of microRNA expression alterations induced by cigarette smoke in mouse lung. Mutat Res 2011;717:9-16. [Crossref] [PubMed]
- Izzotti A, Largheroa P, Balansky R, et al. Interplay between histopathological alterations, cigarette smoke and chemopreventive agents in defining microRNA profiles in mouse lung. Mutat Res 2011;717:17-24. [Crossref] [PubMed]
- Izzotti A, Pulliero A. Molecular damage and lung tumors in cigarette smoke-exposed mice. Ann N Y Acad Sci 2015;1340:75-83. [Crossref] [PubMed]
- Izzotti A, Bagnasco M, Cartiglia C, et al. Chemoprevention of genome, transcriptome, and proteome alterations induced by cigarette smoke in rat lung. Eur J Cancer 2005;41:1864-74. [Crossref] [PubMed]
- Izzotti A, Bagnasco M, Cartiglia C, et al. Modulation of multigene expression and proteome profiles by chemopreventive agents. Mutat Res 2005;591:212-23. [Crossref] [PubMed]
- Schembri F, Sridhar S, Perdomo C, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A 2009;106:2319-24. [Crossref] [PubMed]
- Graff JW, Powers LS, Dickson AM, et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One 2012;7:e44066. [Crossref] [PubMed]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 1992;18:1-29. [Crossref] [PubMed]
- Izzotti A, Calin GA, Steele VE, et al. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev Res (Phila) 2010;3:62-72. [Crossref] [PubMed]
- Izzotti A, Balansky RM, Dagostini F, et al. Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Res 2001;61:2472-9. [PubMed]
- Hecht SS, Trushin N, Rigotty J, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev 1996;5:645-52. [PubMed]
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005;4:1201-15. [Crossref] [PubMed]
- Kang L, Ding L, Wang ZY. Isothiocyanates repress estrogen receptor alpha expression in breast cancer cells. Oncol Rep 2009;21:185-92. [PubMed]
- Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. J Cell Mol Med 2010;14:1485-93. [Crossref] [PubMed]
- Sundar SN, Kerekatte V, Equinozio CN, et al. Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol 2006;20:3070-82. [Crossref] [PubMed]
- Meng Q, Yuan F, Goldberg ID, et al. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr 2000;130:2927-31. [PubMed]
- Izzotti A, Balansky R, D’Agostini F, et al. Modulation by metformin of molecular and histopathological alterations in the lung of cigarette smoke-exposed mice. Cancer Med 2014;3:719-30. [Crossref] [PubMed]
- Kim J, Lee J, Jang S, et al. The anti-estrogen effect of metformin in ERα+ breast cancer and tamoxifen resistant cell lines. Cancer Res 2014;74:Abstract nr 4229.
- Campagnoli C, Berrino F, Venturelli E, et al. Metformin decreases circulating androgen and estrogen levels in nondiabetic women with breast cancer. Clin Breast Cancer 2013;13:433-8. [Crossref] [PubMed]
- Cote ML, Yoo W, Wenzlaff AS, et al. Tobacco and estrogen metabolic polymorphisms and risk of non-small cell lung cancer in women. Carcinogenesis 2009;30:626-35. [Crossref] [PubMed]
- De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol 2013;177:601-12. [Crossref] [PubMed]
- Beattie CW, Hansen NW, Thomas PA. Steroid receptors in human lung cancer. Cancer Res 1985;45:4206-14. [PubMed]
- Balansky R, Ganchev G, Iltcheva M, et al. Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis 2007;28:2236-43. [Crossref] [PubMed]
- Izzotti A, Balansky R, D’Agostini F, et al. Relationships between pulmonary micro-RNA and proteome profiles, systemic cytogenetic damage and lung tumors in cigarette smoke-exposed mice treated with chemopreventive agents. Carcinogenesis 2013;34:2322-9. [Crossref] [PubMed]
- Meireles SI, Esteves GH, Hirata R Jr, et al. Early changes in gene expression induced by tobacco smoke: Evidence for the importance of estrogen within lung tissue. Cancer Prev Res (Phila) 2010;3:707-17. [Crossref] [PubMed]
- Peng J, Xu X, Mace BE, et al. Estrogen metabolism within the lung and its modulation by tobacco smoke. Carcinogenesis 2013;34:909-15. [Crossref] [PubMed]
- Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol 2001;2:506-13. [Crossref] [PubMed]
- Gasperino J, Rom WN. Gender and lung cancer. Clin Lung Cancer 2004;5:353-9. [Crossref] [PubMed]
- Gasperino J. Gender is a risk factor for lung cancer. Med Hypotheses 2011;76:328-31. [Crossref] [PubMed]
- Burns TF, Stabile LP. Targeting the estrogen pathway for the treatment and prevention of lung cancer. Lung Cancer Manag 2014;3:43-52. [Crossref] [PubMed]
- Han W, Pentecost BT, Pietropaolo RL, et al. Estrogen receptor alpha increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1, in normal human bronchial epithelial cells. Mol Carcinog 2005;44:202-11. [Crossref] [PubMed]
- Siegfried JM. Early changes in pulmonary gene expression following tobacco exposure shed light on the role of estrogen metabolism in lung carcinogenesis. Cancer Prev Res (Phila) 2010;3:692-5. [Crossref] [PubMed]
- Li MY, Liu Y, Liu LZ, et al. Estrogen receptor alpha promotes smoking-carcinogen-induced lung carcinogenesis via cytochrome P450 1B1. J Mol Med (Berl) 2015;93:1221-33. [Crossref] [PubMed]
- Stabile LP, Rothstein ME, Cunningham DE, et al. Prevention of tobacco carcinogen-induced lung cancer in female mice using antiestrogens. Carcinogenesis 2012;33:2181-9. [Crossref] [PubMed]
- Cohen A, Smith Y. Estrogen regulation of microRNAs, target genes, and microRNA expression associated with vitellogenesis in the zebrafish. Zebrafish 2014;11:462-78. [Crossref] [PubMed]
- Momi N, Kaur S, Rachagani S, et al. Smoking and microRNA dysregulation: a cancerous combination. Trends Mol Med 2014;20:36-47. [Crossref] [PubMed]
- Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008;40:43-50. [Crossref] [PubMed]
- Chakraborty S, Ganti AK, Marr A, et al. Lung cancer in women: role of estrogens. Expert Rev Respir Med 2010;4:509-18. [Crossref] [PubMed]
- Kumar MS, Lu J, Mercer KL, et al. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet 2007;39:673-7. [Crossref] [PubMed]
- Merritt WM, Lin YG, Han LY, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med 2008;359:2641-50. [Crossref] [PubMed]
- Izzotti A, Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int J Hyg Environ Health 2014;217:601-27. [Crossref] [PubMed]
- Gross TJ, Powers LS, Boudreau RL, et al. A microRNA processing defect in smokers' macrophages is linked to SUMOylation of the endonuclease DICER. J Biol Chem 2014;289:12823-34. [Crossref] [PubMed]
- Shen J, Xia W, Khotskaya YB, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature 2013;497:383-7. [Crossref] [PubMed]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233-47. [Crossref] [PubMed]


