Recurrent left chest mass: a case report
Introduction
Empyema necessitans is a rare complication of untreated pleural space infections leading to spontaneous eruption through the parietal pleura. First described in 1640 by Gullan De Baillon when it developed after the spontaneous rupture of a syphilitic aneurysm, empyema necessitans is a rare entity today. Empyema necessitans was much more common in the pre-antibiotic era; however, with increased use of antibiotics and earlier detection and treatment of pneumonia and parapneumonic effusions, empyema necessitans is rarely seen.
Here we present a rare case of empyema necessitans presenting as an intermittent left chest wall mass that came and went over a three year period before definitive treatment.
Case
Here we describe a 55 year old male with a distant history of large spontaneous bilateral hemothoraces. The patient presented to the Emergency Room in the year 2008 complaining of one week of chest pain and dysphagia. A CT Chest initially suggested a large mass surrounding the esophagus prompting endoscopic evaluation. An endoscopic gastroduodenoscopy was performed where the mucosa was found to be normal appearing, however, a bluish hue was seen at the level of the distal esophagus suggesting blood.
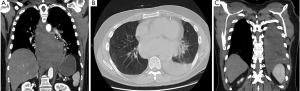
A closer review of the CT Chest (Figure 1A,B,C) revealed a mediastinal hematoma with active extravasation from the right seventh intercostal artery. The patient was taken for embolization and followed for resolution of the hematoma. The patient required bilateral thoracenteses and pigtail catheter placement where over one liter of bloody fluid was removed.
The patient denied any recent trauma or illness and the hemothorax was thought to be spontaneous. The patient was followed until resolution of his symptoms and discharged home.
After discharge the patient was followed by thoracic surgery every six months. On several occasions the patient complained of a mass in his left chest but on repeat exams no mass could be found. Multiple CT scans were performed and no masses could be identified.
Three years after his initial presentation he returned to the Emergency Room with the chief complaint of left back pain and a large mass. The mass was larger than it had been in the past and more painful. Over the last few weeks the mass had begun to grow and cause worsening pain. He denied any fevers or chills, denied night sweats, denied nausea or vomiting, denied diarrhea or constipation, denied shortness of breath or any other complaints.
This patient has a history of diabetes mellitus type 2, major depressive disorder, hyperlipidemia, obsessive compulsive disorder, anxiety seizure disorder, sciatica and a history of poly substance abuse. His surgical history includes left foot surgery, tonsillectomy, embolization of an intracostal artery, thoracenteses bilaterally and skin cancer removal.
The patient has a 25 pack year smoking history, drinks occasionally and uses marijuana regularly.
When examined, the patient had a 15 cm × 15 cm area of induration on his left posterior back with central fluctuance. The area was erythematous and very painful to palpation.
The patient had normal white blood cell count of 10.4, was slightly anemic, with hemoglobin of 13.7 and a hemoglobin A1C of 6.6. The remainder of his labs was within normal limits.
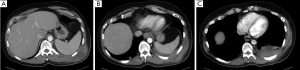
A CT Chest was performed for concerns of abscess or pleural herniation. Figure 2A,B,C shows an empyema and subcutaneous fluid collection in the left hemi-thorax.
The patient was taken to the operating room for exploratory thoracotomy. An incision was made over the fluctuant skin and 300 cc of purulent fluid was expressed. The wound was digitally explored and a 1 cm opening was found in the intercostal space. A thoracotomy was then performed at the level of the opening and an additional 100 cc of purulent material was removed. The cavity within the thorax was loculated, not communicating with the rest of the thorax. A 28 F chest tube was placed into the loculated cavity and the thoracotomy incision was packed with moist gauze. The patient was kept in respiratory isolation until AFB smear and culture resulted negative for tubercle bacilli. On post operative day number one a vacuum closure device was placed over the wound. Wound cultures were negative. The chest tube was removed on post operative day number 3 and the patient was discharged home.
Discussion
In the above case the patient suffered from a rare complication of untreated empyema termed empyema necessitans, where infected fluid dissects spontaneously into the chest wall from the pleural space (1). The most common site of extension is the anterior chest wall because the posterior lung is more adherent. Other sites of extension include esophagus, mediastinum and rarely breast, diaphragm, retroperitoneum and groin (2).
Empyema is a complication a pleural space infection. Five to ten percent of parapneumonic effusions become complicated and lead to empyema (1).
Empyema can be classified as primary thoracic empyema, secondary to lung resection with or without bronchopleural fistula and secondary to trauma (3). Pneumonias are the most common cause of non-traumatic empyema; other causes can be tuberculosis, lung abscess or mediastinitis (4). Thirty percent of all cases originate from thoracic surgical procedures (lung, esophageal, mediastinal or other intrathoracic procedures). About 1.6% to 4.2% of thoracic trauma develops into empyema, most frequently resulting from lung resection with the remainder due to penetrating or blunt chest trauma causing direct contamination or superinfection of retained clots (3).
Empyema evolves through three stages: exudative, fibropurulent and organization (5). In stage I (exudative) the visceral pleura remains elastic and dimensions of the thoracic cavity are unchanged (3). In stage II (transitional or fibrinopurulent) is characterized by turbid and infected fluid, which becomes thick and purulent (3). The fibrin deposits construct bridges which septate the effusions creating multiple loculations (3). In stage III (organizing or consolidative phase) the loculations are replaced by formal granulation tissue (3).
Diagnosis of empyema can be made by the presence of any one of the following: frank purulence on thoracentesis or confirmed organism growth on gram stain or culture; pH below 7.2, glucose less than 400 mg/L, LDH greater the 100 IU/mL, protein >3 g/mL and white blood cell count > 15,000 cells/mm3; physical, radiological, or laboratory signs accompanied by a relevant clinical picture (3).
After antibiotics were introduced in the 1940s, empyema became much less common (6). In the pre-antibiotic era, the majority of cases were associated with mycobacterium tuberculosis, streptococcus pneumoniae, staphylococcus aureus, streptococcus milleri, pseudomonas cepacia and mycobacterium avium-intracellulare (2). Bacteriologic data are sparse in the antibiotic era, but data from the pre-antibiotic era suggest that tuberculosis is the most common cause of empyema necessitans in older patients and pyogenic bacteria in younger patients. Anaerobic bacteria are common, and are cultured in up to 76% of empyemas, usually the result of oropharyngeal aspiration in patients with periodontal disease (5). This is a change from the preantibiotic era, when streptococcus pneumoniae accounted for 60-70% of cases (5). Sometimes, empyema develops or is recognized after the patient has already received antibiotics; culture and gram stain findings may be falsely negative in this situation, such is the case in the patient presented above (1).
Diagnosis is often made radiographically. Computer tomography is the most sensitive modality for differentiating pleural fluid from pleural thickening and for indentifying focal pleural or chest wall abnormalities (5). CXR with lateral decubitus view is used to show presence of loculation or pleural effusions (5). Sonography in the evaluation of superficial masses and pleural space disease is a well established diagnostic modality (7). The presence of echo-free space with a shaggy border in this clinical setting strongly suggested an empyema (7).
Historically, empyema necessitans has become an increasingly rare complication of empyema. Recent case reports have focused on complications associated with empyema necessitans. Contreras et al. reported on a 19 month old male, who presented with empyema necessitans and acute osteomyelitis due to a community-associated, methicillin-resistant staphylococcus aureus strain (2). Dvali et al. described a patient who developed a gas forming soft tissue infection found to be a complication of empyema necessitans (6). A gas-forming cellulitis of the chest wall and left arm, developed one month after the onset of a staphyococcus aureus pneumonia left untreated (6).
Treatment of empyema necessitans includes drainage of purulent collections by thoracotomy, decortication or wide surgical drainage accompanied by antimicrobial therapy (2). Prevention, in the form or early antibiotic therapy for pneumonia will in most cases make the course of the disease uncomplicated and can resolve simple parapneumonic effusions (1). When empyema is suspected, thoracentesis for diagnosis is indicated, followed by drainage with or without irrigation. Tube thoracostomy with a minimum 28 F chest tube can aid in drainage and provide a conduit for irrigation if needed. Instillation of streptokinase, in order to degrade devitalized tissue was described in 1951 (3). It is evident from studies that streptokinase and urokinase decrease empyema viscosity, allow drainage more easily and improve radiologic findings, though its use it not wide spread (1).
Evidence suggests that following a failed tube thoracostomy a VATS evacuation is more beneficial than after an interim attempted fibrinolysis (3). Decortication is the procedure of choice when the underlying lung cannot be expanded with drainage (3). Decortication includes peeling of the organized coat off of the visceral pleura. Marsupialization of the cavity via rib(s) resection and open drainage is a well-established method with low risk. It is the choice of treatment if there is a permanent supply of causative organisms due to bronchopleural fistula (3). Clagett’s procedure consists of open pleural drainage, serial operative debridement and eventual chest closure after filling the pleural cavity with antibiotic solution (3). Weder applies the repetitive thoracotomy and debridement policy with use of regular antiseptic packing (3).
Early identification and treatment of this rare complication of empyema will prevent progression and long term sequelae. Complete evacuation and elimination of the infectious cavity with control of the source organisms form the basis of treatment.
The diagnosis of empyema necessitans should be in the differential diagnosis for any patient presenting with a new thoracic mass following chest trauma or surgery regardless of documented infection.
Conclusions
With the advent of antibiotic therapy and advances in surgical, empyema necessitans is a rarely seen entity. Empyema necessitans usually occurring when patients have limited access to or are reluctant to seek appropriate health care (5). Treatment includes evacuation of the infected space, elimination of the cavity and control of the causative organisms.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ahmed SI, Gripaldo RE, Alao OA. Empyema necessitans in the setting of pneumonia and parapneumonic effusion. Am J Med Sci 2007;333:106-8.
- Contreras GA, Pérez N, Murphy JR, et al. Empyema necessitans and acute osteomyelitis associated with community-acquired methicillin-resistant Staphylococcus aureus in an infant. Biomedica 2009;29:506-12.
- Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiothorac Surg 2007;32:422-30.
- Mehndiratta A, Lawande D, D’Costa L. Empyema Necessitans Following Osteomyelitis of Rib. Ind J Tub 1999;46:45-8.
- Hockensmith ML, Mellman DL, Aronsen EL. Fusobacterium nucleatum empyema necessitans. Clin Infect Dis 1999;29:1596-8.
- Dvali L, Quan C, Pugash R, et al. Empyema necessitans presenting as a gas-forming cellulitis in an HIV-positive man: Case Report. Can J Plast Surg 1999;7:53-6.
- Dershaw DD. Actinomycosis of the chest wall. Ultrasound findings in empyema necessitans. Chest 1984;86:779-80.