Activated RET and ROS: two new driver mutations in lung adenocarcinoma
Introduction
Recent genomic characterization of lung adenocarcinoma led to the discovery of several key genetic alterations involved in the induction of proliferation and metastatic spread as well as in prevention of apoptosis in lung adenocarcinoma cells. Nearly all of these driver mutations are mutually exclusive thus accounting for the classification of lung adenocarcinoma in several genetically defined subgroups. The dependency of the tumors on these driver mutations in the distinct subgroups is underlying their pharmacological vulnerability for specific inhibitors. The identification of specific activating mutations in the EGFR gene and specific rearrangements of the ALK gene have already been successfully translated into clinical routine with the use of in the meantime approved targeted therapeutic agents (erlotinib, gefitinib and crizotinib). Treatment with these targeted therapeutics results in a remarkably increased response rate, progression free survival time and overall survival compared to standard chemotherapy in these molecularly defined subgoups (1-4) thus overcoming for the first time the therapeutic nihilism in advanced adenocarcinoma based on median overall survival times of less than 1 year with chemotherapy unchanged for decades.
Unfortunately, although driver mutations can be identified in over 50% of lung adenocarcinoma (5,6) by now, only 15% of patients with lung adenocarcinoma, i.e. those with EGFR mutations or ALK aberrations, benefit from personalized treatment in clinical routine, while in the other patients either the driver mutations so far have not been fully clinically validated or no driver mutations are known at all.
Recently, two new receptor tyrosine kinase gene rearrangements affecting together up to 3% of lung adenocarcinoma patients were discovered and, based on the observations described in this review, may soon extend the spectrum of effective personalized treatment options in lung cancer.
The rearranged during transfection (RET) gene was found to be rearranged in lung adenocarcinoma patients (1%) for the first time in 2012 by four independent groups (7-10) and preliminary studies demonstrated sensitivity of lung cancer cell lines harboring a RET rearrangement to RET-kinase inhibitors like vandetanib (9).
The ROS1 rearrangement was first discovered in lung adenocarcinoma in 2007 (11). In 2012 a study determined a frequency of ROS1 rearrangements in a large lung adenocarcinoma cohort (n=1,073) of 2% (12). In addition, first results of a phase I trial investigating the use of crizotinib in patients harboring ROS1 rearrangements showed promising results (13).
Both kinases are involved in rearrangements resulting in fusion of their kinase domains to different partners. The fusion partners are responsible for the homo-dimerization underlying the oncogenic potency of the gene fusion products.
This review will focus on RET- and ROS1 kinases, their physiological role in the cell and their function as an oncogenic driver especially in lung adenocarcinoma. Furthermore, we will give an overview on current RET- and ROS1 kinase inhibitors and current clinical trials evaluating specific RET- and ROS1 inhibitors.
RET discovery and mechanism of action
The RET proto-oncogene was first described as an oncogene activated through DNA rearrangement in the NIH-3T3 cell model in 1985 (14). RET is located on chromosome 10q11.2 and spans 21 exons. It encodes for a receptor tyrosine kinase with an extracellular domain (containing four cadherin-like repeats, a calcium binding site, and a cystein rich region), a transmembrane region and an intracellular kinase domain (15). There are three common isoforms of RET, the long (RET51), intermediate (RET43), and short (RET9) form, which arise through alternative splicing of the mRNA at the carboxyterminal cytoplasmic tail. They are named after the number of amino acids that follow the point of divergence. RET51 and RET9 are the best characterized isoforms (16). The main ligands of the RET protein belong to the glial-derived neurotrophic factor (GDNF) family, which include GDNF, artemin, neurturin and persephin. The RET-Receptor is part of a cell surface complex, it binds a member of the GDNF family in conjunction with GDNF-family receptor alpha (GFR) co-receptors. After a ligand has bound to the RET-Receptor, it is activated through the formation of a RET-homodimer with subsequent activation of the kinase domain leading to autophosphorylation of intracellular domains (17). Multiple downstream signaling pathways are activated through the activation of the RET protein, these include the RAS/RAF/ERK, the PI3K/AKT and the JNK pathways (18). RET is expressed in neuronal subsets of the central and peripheral nervous system, the Wolffian duct, the budding ureter, the nephric duct and spermatogonia. RET kinase null mice are born alive, but die within one day because of renal aplasia or dysplasia. They also do not develop enteric nervous plexuses, which is in line with the development of Hirschprung’s disease caused by loss-of-function mutations in RET (19).
The first link between RET and human cancer was established by the discovery of somatic rearrangements of RET in papillary thyroid carcinoma (RET/PTC). These rearrangements lead to a constitutive activation of the tyrosine kinase (18). Up to 30% of sporadic and up to 70% of radiation induced papillary thyroid carcinomas (PTC) show a somatic rearrangement of the RET gene (20). So far 12 different 5’-fusion partner genes of RET have been described. Germline activating point mutations of RET are associated with the multiple endocrine neoplasia type 2 (MEN 2) syndrome. The MEN 2 syndrome is divided into three distinct phenotypes: MEN 2A [medullary thyroid carcinoma (MTC), pheochromacytoma (PC) and hyperparathyroidism], MEN 2B (MTC and PC) and Familial Medullary Thyroid Cancer (FMTC). Each phenotype has a strong association with specific mutation sites within the RET gene. Somatic mutations in RET are also associated with 50% of sporadic medullary thyroid cancer (21).
RET in NSCLC
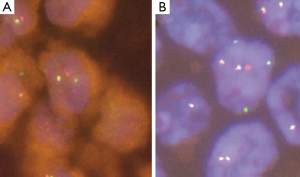
In 2012 four independent groups identified independently the presence of a new rearrangement involving the RET-kinase domain in NSCLC. These groups screened a total of 2,650 lung cancer patients (22) and described a frequency of 1% RET rearrangements (7-9). RET expression in the lung is under normal conditions very low, but is significantly increased with the presence of the RET fusion gene (7). The transforming ability of the KIF5B-RET fusion gene could be shown in preclinical models using Ba/F3 cells, which were shown to grow interleukin-3 independent after expression of the fusion protein and in NIH 3T3 cells, which showed anchorage-independent cell proliferation after the expression of the RET fusion protein (7). The artificial cell systems showed sensitivity to the treatment with the multi-kinase inhibitors vandetanib, sunitinib and sorafenib, which are able to inhibit the kinase activity of RET (9). Current data suggests, that RET rearrangements occur mutually exclusive with other known driver alterations in NSCLC, which further supports its role as a driving oncogene in NSCLC (9,22). The most common RET fusion protein is comprised of the first 15 exons of the KIF5B gene and the exons 12-20 of the RET gene. Exon 1-15 of KIF5B contains a kinesin motor and coiled-coil domains that mediate homodimerization of the fusion proteins (9). The KIF5B exon 15 fusion site was also shown to be present in the KIF5B-ALK fusion protein in NSCLC (23). Exons 12-20 of the RET gene contain the RET kinase domain allowing downstream kinase signaling and activation of the PI3K/Akt and/or the RAS/MAPK pathway (24). Up till now 7 variants of the KIF5B-RET fusion gene have been described and besides KIF5B two other fusion partner have been detected, i.e. the CCDC6 gene and the NCOA4 (nuclear receptor coactivator 4) gene (8,25). CCDC6 and NCOA4 have been described before in PTC as RET/PTC1 and RET/PTC3, respectively. They both also contain coiled-coil domains, which are able to mediate dimerization of the oncoproteins (26). Concerning the correlation between NSCLC harboring RET fusion and clinical characteristics, Wang and colleagues screened 936 patients with NSCLC and could identify 13 RET fusion positive patients (1.4%) in their population. They suggested specific clinicopathologic characteristics for RET fusion positive patients, including younger age (≤60 years), never-smoker status, early lymph-node metastases, poor differentiation of the tumor and a solid predominant subtype (25). The detection of fusion genes can be conducted using RT-PCR, FISH and IHC. Interesting data have been published showing a sensitivity of 90% and specifity of 97.8% for IHC testing for ALK translocations, when compared to FISH in NSCLC (27). So far, however, IHC has not been established for RET detection and thus was not used in trials screening for RET fusions in NSCLC (9,25). Therefore FISH, although cost and labor intensive, still seems to be the gold standard for the detection of RET fusions (Figure 1) in NSCLC. In addition, the use of RT-PCR might miss new fusion partners (25). However, given the necessity of simultaneous pretherapeutic assessment of numerous driver mutations in lung cancer, it seems reasonable that next generation based multiplex sequencing will substitute distinct single gene assays involving RET in the near future (28).
RET inhibitors
So far no clinical trials using RET inhibitors in NSLCL harbouring RET fusion genes have been published. Since RET fusions and activating mutations are present in differentiated thyroid carcinomas (DTC) and medullary thyroid carcinoma (MTC) (20) we will thus start to summarize data from clinical trials studying tyrosine kinase inhibitors with anti-RET activity in patients with DTC and MTC. However, these results may only be of limited value for the understanding of RET inhibition in NSCLC, since the spectrum of RET mutations and RET fusions in thyroid carcinoma differs from what so far is known for RET in NSCLC.
Specific aberrations in RET present in thyroid carcinoma can be assigned to specific subgroups of the disease. RET fusions are mostly present in PTC, which closely resemble the fusions present in NSCLC (8,26). Preclinical studies have shown that different tyrosine kinase inhibitors with anti-RET activity show different activity against the various aberrant RET forms present in thyroid carcinomas (29). For instance, cabozantinib showed greater activity compared to vandetanib in cells harboring the RET/PTC1 fusion gene (29), a fusion gene which is also present in NSCLC. These observations should be considered in planning trials with anti-RET tyrosine kinase inhibitors also in lung cancer.
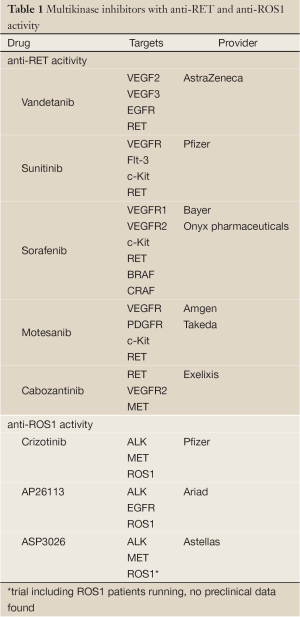
Anti-RET tyrosine kinase inhibitors have already been evaluated in NSCLC, however, not focusing of patients harbouring RET fusion genes in their tumors (30-32). Thus, these trials do not add valuable information concerning the use of these drugs in RET positive lung cancer patients only. Furthermore, the drugs used in these trials (vandetanib, sunitinib, sorafenib) are no specific RET inhibitors, but rather multi kinase inhibitors. This fact further complicates the interpretation of RET-inhibition in thyroid carcinoma (24). For a list of multi-kinase inhibitors with anti-RET activity refer to Table 1.
Full Table
Vandetanib
In April 2011 the FDA approved vandetanib, a RET, VEGF 2, VEGF 3 and EGF receptor tyrosine kinase inhibitor for the treatment of patients with metastatic MTC who were ineligible for surgery and had progressive or symptomatic disease. The approval followed the results from an open label single arm phase II study testing vandetanib in patients with hereditary MTC. This phase II study conducted by Wells et al. showed that 83% of the patients treated with vandetanib had a reduction in tumor size at their first assessment and 11 out of 30 patients responded with an initial decrease in tumor size ≥30% of which 6 (20%) had confirmed partial responses (PR) according to RECIST. Disease control rate at 24 weeks was 78% and the duration of response in patients with confirmed PR was durable with a median of 10.2 months (33). Following the phase II data a large phase III trial was initiated showing a significantly improved efficacy and prolongation of PFS for vandetanib compared to placebo in patients with sporadic and hereditary MTC with a hazard ratio of 0.46 (95% CI, 0.31-0.69; P<0.001) (34). Preclinical studies suggest that vandetanib has superior activity in MEN2B cell lines compared to cabozantinib (29). The predominant mutation in MEN2B is the activating M918T point mutation in the RET kinase domain, which is also the most frequent mutation in sporadic MTC (35). Vandetanib also showed activity against RET/PCT in vitro and in vivo (36).
Cabozantinib
Cabozantinib, a potent inhibitor of RET, VEGFR2 and MET tyrosine kinases, received FDA approval for its use in MTC in November 2012. Early signals of activity in MTC were seen in a phase I dose escalation trial, which led to the testing of cabozantinib in patients with MTC in an expansion cohort of the phase I study. Of the 35 patients with MTC and measurable disease included into the study 17 patients (49%) experienced a 30% or greater reduction in the sum of tumor diameters at first assessment. Disease control of at least 6 months was present in 68% of the patients (37). Following the positive data from the phase II study a large phase III study was started, which tests cabozantinib vs. placebo in patients with progressive, unresectable, locally advanced or metastatic MTC. First data were presented at ASCO 2012, which showed that the primary objective of significant PFS prolongation was met (HR 0.28 95% CI, 0.19-0.40; P<0.0001) (38).
In July 2012 a phase II study testing cabozantinib in KIF5B/RET positive NSCLC patients has been initiated at Memorial Sloan-Kettering Cancer Center (NCT01639508) and is thus to our knowledge the first study investigating a personalized treatment approach for this newly defined subgroup of NSCLC. Interestingly, in vitro studies showed a greater activity of cabozantinib compared to vandetanib in cell lines harboring the RET/PTC1 fusion gene, which also has been found in NSCLC (29).
Sorafenib
Sorafenib is a multi-tyrosine kinase inhibitor targeting VEGFR1, VEGFR2, KIT, RET, BRAF and CRAF (39). In vitro sorafenib was shown to inhibit RET in the low nanomolar range and exerted anti-tumor activity in RET-driven xenografts (40). Sorafenib has been tested in several phase II studies in patients with DTC, anaplastic thyroid carcinoma and MTC (41-43). In an open-label phase II study of 41 patients with PTC, 6 patients (15%) showed a PR and 23 (56%) patients had a stable disease for longer than 6 months. The PRs seen in the patients were durable with a median duration of 7.5 months. The authors concluded that sorafenib is an active drug in metastatic PTC. Genetic testing was included into the trial and the great majority of PTCs harbored an activating BRAF mutation whereas none was positive for RET/PTC1 or RET/PTC3. These observations render translation into the RET driven NSCLC setting difficult (43). In another phase II study sorafenib was tested in locally advanced or metastatic MTC. Of 15 evaluable patients with sporadic MTC, one patient had a PR and more than 50% of the patients had SD ≥15 months. The majority of tumors in the tested population had activating mutations in the RET gene (42) The phase II study from Gupta-Abramson et al. demonstrated in 30 (27 out of 30 being DTC) patients with metastatic, iodine-refractory thyroid carcinoma a PR rate of 23% (7 patients). The median PFS was stated with 19.75 months. Data of specific genetic testing were not presented in this paper. Given the PR rate of 23% and the PFS of 19.25 months the sorafenib treatment may be considered superior to chemotherapy in these patients (41).
Sunitinib
Sunitinib is a multi-tyrosine kinase inhibitor targeting VEGFR, Flt-3, c-Kit and RET (40) and has proven to be a potent inhibitor of RET/PTC oncoproteins in vitro and in vivo (36). In a phase II study in iodine refractory DTC and MTC from 33 evaluable patients one patient showed a complete response (3%), ten patients had a PR (28%) and 16 patients demonstrated stable disease (46%). There was also a significant association seen between decreased 18FDG-PET uptake and RECIST response (44). Intermediate results of two studies testing sunitinib in patients with thyroid carcinoma were presented at ASCO 2008 (45,46). The study of Cohen et al. presented data of 31 evaluable patients with DTC treated for at least two cycles with sunitinib. Of these patients 13% showed a PR and 65% of patients a SD. In MTC there have been no PRs reported, but a SD rate of 85% (45) In a mixed patient cohort with MTC, DTC and anaplastic thyroid carcinoma Ravaud et al. demonstrated in 15 evaluable patients a PR rate of 7% (n=1) and a SD rate of 80% (n=12) (46). In addition two case reports have been published, one reporting a PR in a patient with MTC and one in a patient with PTC treated with sunitinib (47,48).
Motesanib
The multi-tyrosine kinase inhibitor motesanib inhibits VEGFR, PDGFR, Kit and RET and demonstrated activity in TT tumor cell xenografts expressing the RET C634W protein (49). But there have also been reports indicating the ineffectiveness of motesanib in inhibiting the C634W mutant form of RET and being only active in wild type RET (50). Motesanib was tested in two phase II studies involving patients with thyroid cancer. One study which included 93 patients with confirmed locally advanced metastatic DTC or MTC yielded a 14% PR rate and a 68% SD rate. However none of the patients genetically analyzed showed a RET mutation or RET rearrangement in their tumor (51). Another phase II trial studying motesanib in MTC included 91 patients. In this trial only 2% of the patients were reported to have achieved a PR and 81% of the patients had a SD. The objective response rate for RET-mutation negative (n=10) and for RET-mutation positive (n=28) was 10% and 0%, respectively (50).
ROS1 discovery and mechanism of action
ROS was first described as an oncogene product of the avian sarcoma RNA tumor virus UR2 (University of Rochester tumor virus 2) in 1982 and v-ROS1 was identified in the same year as the distinctive oncogenic sequence in UR2 (52,53). In the UR 2 virus v-ROS1 is fused to the gag-gene and the product of the fusion gene gag-ros was identified to have tyrosine kinase activity (54). In 1984 the mf3 gene, which later was discovered to be similar to c-ROS1, was reported to induce malignant transformation of NIH3T3 cells (55). ROS1 (v-ros avian UR2 sarcoma virus oncogene homolog 1) has been mapped to chromosome 6q16-6q22 (56). The region is involved in nonrandom chromosomal rearrangements in different malignancies including, glioblastoma, cholangiocarcinoma and lung adenocarcinoma (8,57,58). The ROS1 receptor tyrosine kinase consists of an extracellular domain, a hydrophobic transmembrane region and an intracellular kinase domain. ROS is a unique receptor which is remotely related to the ALK and Insulin receptor family (59,60). The extracellular domain of ROS1 contains a YWTD -propeller domain that folds into three -propeller domains and nine fibronectin type III domains. Although ROS1 has a large extracellular domain no ligand has been found so far. The presence of fibronectin III domains is a common feature of cell adhesion molecules (CAMs), therefore the combination of fibronectin III domains in the extracellular domain of ROS1 coupled with the intracellular kinase activity might be a way of direct translating adhesion engagements to intracellular signaling pathways (60). Multiple downstream signaling pathways are activated through the activation of ROS1, these include the STAT3, PI3K/AKT and RAS/MAPK/MEK pathway, although it is important to notice that the transforming ability of the chimeric EGFR-ROS on chicken embryo fibroblasts or NIH3T3 cells was not hindered through the application of the MEK inhibitor PD98059 (61). ROS1 was shown to be expressed in mouse, rat and chicken kidneys and intestine (60). In mice the expression of c-ROS seems to play a role in the development of the kidneys, especially in stages which involve epithelial-mesenchymal interactions. In adult mice with mature kidneys c-ROS expression is low (62). In the testicles expression of c-ROS was detected in mice and is limited to epithelial cells of the caput epididymis. The importance of ROS for the maturation of the epithelial cells of the epididymis was seen in ROS-null mice. These mice lost the ability of reproduction due to deficient sperm function, which was most likely due to improper capacitation supporting the significance of a functional epididymis for maturation of the spermatocytes. Besides from infertility c-ROS knockout mice were healthy (63). In humans c-ROS has also been detected in the epididymis, although the spatial distribution was different from what was seen in mice (64). Expression of c-ROS was also detected in other human tissues, such as lung, placenta and skeletal muscle tissue (60).
The first link between human cancer and ROS1 was established in 1987 by the discovery of somatic rearrangements involving ROS1 in glioblastoma cell lines, although the partner of ROS1 was not identified (65). The fusion of the FIG (Fused in Glioblastoma)-gene with ROS1 was elucidated by Charest et al. in 2003. It was shown that the FIG-ROS1 fusion protein was created through a small intra-chromosomal deletion and was therefore the first example of a receptor tyrosine kinase fusion protein, which did not occur from a translocation or inversion (57). Furthermore it was shown that the FIG-ROS fusion protein was able to transform NIH3T3 cells in vitro and to enable tumor formation in immunocompromised nude mice (58). The expression of the FIG-ROS fusion protein in the CNS in vivo was able to induce the formation of glioblastomas (66). FIG-ROS fusion proteins were also discovered in cholangiocarcinoma cell lines and patient derived tumors (58).
ROS1 in NSCLC
Rearrangements in NSCLC involving ROS1 (Figure 1) were first described by Rikova et al. in 2007.
In a phosphoproteomic screen of 41 NSCLC cell lines and 150 NSCLC tumors 2 ROS1 fusions (SLC34A2-ROS1 and CD74-ROS1) were detected (11). SLC34A2-ROS1 was discovered in the HCC78 cell line. SLC34A2 is part of the solute carrier family and is expressed in many different organs such as lung, mammary glands, testis and liver. It is believed that the gene product of SLC34A2 the protein NaPi-IIb is involved in the reabsorbtion of Pi in the surfactant of lung alveolars. The protein is supposed to span the cell membrane in 8 loops (67). Mutations of SLC34A2, which abrogate the normal protein function, are associated with pulmonary alveolar microlithiasis (68). In the fusion gene 2 variants exist, either a fusion between exon 4 of SLC34A2 and exon 32 of ROS or exon 4 of SLC34A2 and exon 34 of ROS. In both cases the fusion gene expresses a protein with two transmembrane domains (11). The CD74-ROS1 fusion was discovered in a tumor from a female never-smoker with adenocarcinoma. In this tumor exon 6 of CD74 was found to be fused with exon 34 of ROS1. CD74 codes for a type II membrane protein. The protein functions as a receptor for the macrophage migration inhibitory factor and as a chaperon for MHC class II proteins (69). The transforming ability of SLC34A2-ROS was shown in the ability of the fusion gene to cause anchorage-independent growth and tumor formation in nude mice of 3T3 cells transduced with a retrovirus containing SLC34A2-ROS (58). The oncogenic ability of the CD74-ROS1 fusion gene has also been validated in fibroblasts and NSCLC cells were the ectopic expression of the CD74-ROS1 fusion gene induced high invasiveness in vitro (Matrigel Boyden chamber invasion assay) and the formation of metastases in vivo (70).
Recently, two large studies screening together more than 2000 patients with NSCLC for the presence of ROS1 rearrangements found the frequency of ROS1 rearrangements in NSCLC to be approximately 2% (8,12). The clinical characteristics of patients with a ROS1 fusion were very similar to patients with an ALK translocation. It was found that patients with a ROS1 fusion positive tumor were more commonly light smokers (<10 pack years) or never-smokers and ROS1 fusions were associated with younger age and adenocarcinoma histology (12). The study conducted by Takeuchi et al. and Govindan et al. discovered additional fusion partners of ROS1 in NSCLC: tropomyosin 3 (TPM3), syndecan 4 (SDC4), leucine-rich repeats and immunoglobulin-like domains (LRIG3), ezrin (EZR) and endoplasmic reticulum protein retention receptor 2 (KDELR 2) (8,71). The kinase activity of ROS1 is retained in the known fusion proteins (72) and ROS1 rearrangements were not overlapping with other known oncogenic events in NSCLC, like KRAS mutations, EGFR mutations or ALK fusions (12). A transgenic mouse model expressing the EZR-ROS1 fusion protein in lung alveolar epithelial cells has been developed and could demonstrate the formation of adenocarcinoma in both lungs at an early age (73).
ROS1 inhibitors
Although ROS1 has been known to play a role as an oncogene in glioblastoma for a long time (65), selective ROS1 inhibitors have not yet been clinically tested. Given that the ROS1 kinase shares high sequence homology with ALK, which is reflected in an amino acid sequence homology of 77% at the adenosine triphosphate (ATP)-binding site (74), the activity of ALK-kinase inhibitors were tested in cell lines and tumors harboring ROS1 fusion proteins (58). The ALK-inhibitor TAE684 showed activity in the lung cancer cell line HCC78, which harbors the SLC34A2-ROS1 fusion gene and in BaF3 cells expressing the FIG-ROS fusion protein (58,75). Crizotinib, the approved ALK/MET inhibitor for NSCLC patients harboring an ALK-translocation also showed activity in the HCC78 cell line (12,76). Following these signals the phase I trial of crizotinib (NCT00585195) was amended for the inclusion of patients with solid tumors harboring a ROS1 rearrangement. Preliminary results presented at ASCO and ESMO 2012 demonstrated promising results of crizotinib in ROS1 rearranged NSCLC with an objective response rate of 57% and a disease control rate of 80% after 2 months (13). Our group also recently published a case of a heavily pretreated NSCLC patient whose tumor harbored a ROS1 rearrangement and showed a compete metabolic response in 18FDG-PET/CT after 4 weeks of treatment with crizotinib which is maintained now for more than 4 months (77). Currently there are also two trials ongoing testing second-generation ALK inhibitors in ROS1 fusion positive tumors. These trials are evaluating the safety and activity of AP26113 (NCT01449461) and ASP3026 (NCT01284192). For a list of multi-kinase inhibitors with anti-ROS1 activity refer to Table 1.
Conclusions
The identification of RET- and ROS1 rearrangements in NSCLC is a consequence of our increasing knowledge of the genomic basis of malignant transformation in lung cancer resulting in the identification of an increasing number of distinct and therapeutically tractable molecular subgroups. Tyrosine kinase inhibitors with anti-RET activity have shown promising preclinical and clinical activity in thyroid carcinomas. However, up till now most of the trials conducted were entity driven and did not distinguish between the molecular subtypes which are present in thyroid carcinomas and NSCLC. Thus, although these trials provide some evidence that aberrant RET may serve as a target for kinase inhibitor therapy, the translation of these observations to NSCLC seems to be problematic. Therefore, prospective trials RET translocated NSCLC are needed, although their realization may be a challenge given the low incidence of RET rearrangements in NSCLC. The ongoing trial of cabozantinib in KIF5B-RET fusion positive NSCLC (NCT01639508) is a first step in the right direction. In the case of ROS1 an impressive activity of the ALK/MET/ROS inhibitor crizotinib has already been reported as result of a still ongoing phase I trial in heavily pretreated NSCLC patients with ROS rearrangements in their tumors. Based on these results approval of crizotinib for the treatment of ROS positive NSCLC in the near future seems probable. Other clinical trials evaluating the safety and activity of second generation small molecule inhibitors with anti-ROS1 activity are also currently tested in ROS1 fusion positive patients, but no results have been presented so far. Furthermore, it remains to be elucidated how new selective RET- and ROS-inhibitors will perform clinically (78). Given the low frequency of these two new driver mutations the execution of clinical trials addressing the efficacy of RET- and ROS-inhibitors is in particular challenging and requires the establishment of large and effective molecular screening networks providing real time molecular diagnostics of high quality (28).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703.
- Kim D, Ahn M, Yang P, et al. Results of a Global Phase II Study with Crizotinib in Advanced ALK-positive Non-small Cell Lung Cancer (NSCLC). ASCO Annual Meeting 2012;2012:ix400-46.
- Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol 2011;22:2616-24.
- Zander T, Heukamp LC, Bos MA, et al. Regional screening network for characterization of the molecular epidemiology of non-small cell lung cancer (NSCLC) and implementation of personalized treatment. J Clin Oncol 2012;30:abstr CRA10529.
- Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375-7.
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81.
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382-4.
- Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res 2012;22:436-45.
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203.
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70.
- Shaw AT, Camidge DR, Engelman J, et al. Clinical activity of crizotinib in patients with advanced non-small cell lungcancer (NSCLC) harboring ROS1 gene rearrangement. Ann Oncol 2012;30:abstr 7508.
- Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 2001;12:361-73.
- Ishizaka Y, Itoh F, Tahira T, et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989;4:1519-21.
- Myers SM, Eng C, Ponder BA, et al. Characterization of RET proto-oncogene 3' splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene 1995;11:2039-45.
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 2002;3:383-94.
- Phay JE, Shah MH. Targeting RET receptor tyrosine kinase activation in cancer. Clin Cancer Res 2010;16:5936-41.
- Manié S, Santoro M, Fusco A, et al. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet 2001;17:580-9.
- Jhiang SM. The RET proto-oncogene in human cancers. Oncogene 2000;19:5590-7.
- Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 2008;21:S37-43.
- Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat Med 2012;18:349-51.
- Wong DW, Leung EL, Wong SK, et al. A novel KIF5B-ALK variant in nonsmall cell lung cancer. Cancer 2011;117:2709-18.
- Chau NG, Haddad RI. Vandetanib for the treatment of medullary thyroid cancer. Clin Cancer Res 2013;19:524-9.
- Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352-9.
- Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol 2006;155:645-53.
- Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459-65.
- Buettner R, Wolf J, Thomas RK. Lessons to learn from lung cancer genomics: The emerging concept of individualized diagnostics and treatment. J Clin Oncol 2013. [Epub ahead of print].
- Verbeek HH, Alves MM, de Groot JW, et al. The effects of four different tyrosine kinase inhibitors on medullary and papillary thyroid cancer cells. J Clin Endocrinol Metab 2011;96:E991-5.
- Novello S, Camps C, Grossi F, et al. Phase II study of sunitinib in patients with non-small cell lung cancer and irradiated brain metastases. J Thorac Oncol 2011;6:1260-6.
- Dy GK, Hillman SL, Rowland KM, et al. A front-line window of opportunity phase 2 study of sorafenib in patients with advanced nonsmall cell lung cancer: North Central Cancer Treatment Group Study N0326. Cancer 2010;116:5686-93.
- Lee JS, Hirsh V, Park K, et al. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol 2012;30:1114-21.
- Wells SA Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 2010;28:767-72.
- Wells SA Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41.
- Frank-Raue K, Rondot S, Raue F. Molecular genetics and phenomics of RET mutations: Impact on prognosis of MTC. Mol Cell Endocrinol 2010;322:2-7.
- Kim DW, Jo YS, Jung HS, et al. An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. J Clin Endocrinol Metab 2006;91:4070-6.
- Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660-6.
- Schoffski P, Elisei R, Muller S. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. ASCO Meeting 2012;34:5508.
- Morgillo F, Martinelli E, Troiani T, et al. Antitumor activity of sorafenib in human cancer cell lines with acquired resistance to EGFR and VEGFR tyrosine kinase inhibitors. PLoS One 2011;6:e28841.
- Mologni L. Development of RET kinase inhibitors for targeted cancer therapy. Curr Med Chem 2011;18:162-75.
- Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714-9.
- Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010;28:2323-30.
- Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009;27:1675-84.
- Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 2010;16:5260-8.
- Cohen E, Needles B, Cullen K. Phase 2 study of sunitinib in refractory thyroid cancer. J Clin Oncol. 2008;26:abstr 6025.
- Ravaud A, De la Fouchardière C, Courbon F. Sunitinib in patients with refractory advanced thyroid cancer. J Clin Oncol 2008;26:abstr 6058.
- Kelleher FC, McDermott R. Response to sunitinib in medullary thyroid cancer. Ann Intern Med 2008;148:567.
- Dawson SJ, Conus NM, Toner GC, et al. Sustained clinical responses to tyrosine kinase inhibitor sunitinib in thyroid carcinoma. Anticancer Drugs 2008;19:547-52.
- Coxon A, Bready J, Kaufman S, et al. Anti-tumor activity of motesanib in a medullary thyroid cancer model. J Endocrinol Invest 2012;35:181-90.
- Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II Study of Safety and Efficacy of Motesanib in Patients With Progressive or Symptomatic, Advanced or Metastatic Medullary Thyroid Cancer. J Clin Oncol 2009;27:3794-801.
- Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 2008;359:31-42.
- Wang LH, Hanafusa H, Notter MF, et al. Genetic structure and transforming sequence of avian sarcoma virus UR2. J Virol 1982;41:833-41.
- Shibuya M, Hanafusa H, Balduzzi PC. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol 1982;42:143-52.
- Feldman RA, Wang LH, Hanafusa H, et al. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol 1982;42:228-36.
- Fasano O, Birnbaum D, Edlund L, et al. New human transforming genes detected by a tumorigenicity assay. Mol Cell Biol 1984;4:1695-705.
- Nagarajan L, Louie E, Tsujimoto Y, et al. The human c-ros gene (ROS) is located at chromosome region 6q16----6q22. Proc Natl Acad Sci U S A 1986;83:6568-72.
- Charest A, Lane K, McMahon K, et al. Fusion of FIG to the receptor tyrosine kinase ROS in a glioblastoma with an interstitial del(6)(q21q21). Genes Chromosomes Cancer 2003;37:58-71.
- Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One 2011;6:e15640.
- Birchmeier C, O’Neill K, Riggs M, et al. Characterization of ROS1 cDNA from a human glioblastoma cell line. Proc Natl Acad Sci USA 1990;87:4799-803.
- Acquaviva J, Wong R, Charest A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim Biophys Acta 2009;1795:37-52.
- Nguyen KT, Zong CS, Uttamsingh S, et al. The role of phosphatidylinositol 3-kinase, rho family GTPases, and STAT3 in Ros-induced cell transformation. J Biol Chem 2002;277:11107-15.
- Tessarollo L, Nagarajan L, Parada LF. c-ros: the vertebrate homolog of the sevenless tyrosine kinase receptor is tightly regulated during organogenesis in mouse embryonic development. Development 1992;115:11-20.
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, et al. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev 1996;10:1184-93.
- Légaré C, Sullivan R. Expression and localization of c-ros oncogene along the human excurrent duct. Mol Hum Reprod 2004;10:697-703.
- Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci USA 1987;84:9270-4.
- Charest A, Wilker EW, McLaughlin ME, et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res 2006;66:7473-81.
- Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch 2004;447:763-7.
- Tachibana T, Hagiwara K, Johkoh T. Pulmonary alveolar microlithiasis: review and management. Curr Opin Pulm Med 2009;15:486-90.
- Borghese F, Clanchy FIL. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin Ther Targets 2011;15:237-51.
- Jun HJ, Johnson H, Bronson RT, et al. The oncogenic lung cancer fusion kinase CD74-ROS activates a novel invasiveness pathway through E-Syt1 phosphorylation. Cancer Res 2012;72:3764-74.
- Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121-34.
- Stumpfova M, Jänne PA. Zeroing in on ROS1 rearrangements in non-small cell lung cancer. Clin Cancer Res 2012;18:4222-4.
- Arai Y, Totoki Y, Takahashi H, et al. Mouse Model for ROS1-Rearranged Lung Cancer. PLoS One 2013;8:e56010.
- Chin LP, Soo RA, Soong R, et al. Targeting ROS1 with anaplastic lymphoma kinase inhibitors: a promising therapeutic strategy for a newly defined molecular subset of non-small-cell lung cancer. J Thorac Oncol 2012;7:1625-30.
- McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 2008;68:3389-95.
- Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, et al. Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol 2012;7:1086-90.
- Bos M, Gardizi M, Schildhaus HU. Complete metabolic response in a patient with repeatedly relapsed non-small cell lung cancer harboring ROS1 gene rearrangement after treatment with crizotinib. Lung Cancer in press.
- El-Deeb IM, Yoo KH, Lee SH. ROS receptor tyrosine kinase: a new potential target for anticancer drugs. Med Res Rev 2010. [Epub ahead of print].


