Immunotherapy and radiation therapy for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive malignant neoplasm arising from the mesothelial lining of the pleura. MPM is a relatively rare malignancy and annual incidence varies between countries: 29 per 1,000,000 people per year in Australia and the UK, 10 per 1,000,000 people per year in the USA, and eight per 1,000,000 people per year in Japan (1). MPM is primarily caused by inhalation of asbestos fibers, and exposure to asbestos can be documented in up to 80% of cases (2). More recently, familial predisposition for the development of mesothelioma has been shown to be associated with germline BAP1 gene mutations (3). The histologic subtypes of MPM include epithelioid (approximately 60%) and non-epithelioid types (approximately 40%); non-epithelioid variants include the subtypes of sarcomatoid, spindle, desmoplastic, fibrous, biphasic, and not otherwise specified (4). In general, MPM is considered to be an incurable malignancy and is often diagnosed at an advanced stage.
The current available treatments for MPM include resection with the intent to achieve a gross total resection, radiation, and systemic chemotherapy. Surgical therapy is a viable option in a minority of patients, with only 10–15% of patients presenting as medically operable and at a stage where resection is feasible (5). The generally preferred surgical procedure for resection is extended pleurectomy and decortication (EP/D). Prior studies evaluating extrapleural pneumonectomy (EPP) have failed to demonstrate a significant survival benefit (6). Although underpowered for survival, the MARS trial suggested that EPP was not better, and may be inferior, to standard chemotherapy (7). Studies of EP/D as the primary surgical modality have generally shown less operative morbidity/mortality than EPP. The reported median overall survival ranges between 13–29 months for EP/D and 12–22 months for EPP, with a trend favoring EP/D (8), with even more extended survival in select EP/D series. However, the benefits of surgical resection may be limited to patients with epithelioid histology (9,10).
The standard first-line chemotherapy regimen is the combination of cisplatin and pemetrexed, which is associated with a median overall survival of about 12 months (11). More recently, the addition of anti-angiogenic therapy to chemotherapy demonstrated improvement in overall survival outcomes. In a phase III randomized trial, the addition of bevacizumab to cisplatin/pemetrexed significantly improved the median overall survival compared to chemotherapy alone, 18.8 vs. 16.1 months, respectively (12). There are no FDA-approved therapies for subsequent therapy. Single-agent chemotherapy with gemcitabine or vinorelbine is commonly used, although response rates are around 10% (13). Re-treatment with single-agent pemetrexed has also shown benefit in patients who have demonstrated disease control of at least 12 months with initial therapy (14).
Immunotherapy for mesothelioma
The immune system plays an important role in cancer surveillance and tumor rejection (15). Evidence of this role can be seen in cases of spontaneous tumor regression, the regression of metastases after removal of the primary tumor, infiltration of tumors by immune effector cells, and higher incidence of cancers in immunocompromised patients. Immunotherapy of cancer has come to forefront with the discovery of novel targets of immune regulation; however, immunotherapy is certainly not a new concept. The first attempts at immunotherapy for cancer date back to the 1890s when Dr. William Coley demonstrated regression of tumors with the injection of heat-killed preparations of Strep. pyogenes and Serratia marcescens (16). While “Coley’s toxin” was never used for mesothelioma, infection has been reported as a trigger for spontaneous regression of thoracic malignancies (17).
Broader applications of immunotherapy became possible with the discovery of cytokines in the 1950s, including interferons and interleukins (18). For example, interleukin-2 (IL-2) has been shown to activate cytotoxic T-lymphocytes and natural killer (NK) cells and is clinically active in the treatment of advanced melanoma and renal cell carcinoma (19). The toxicity of IL-2 limits therapeutic efficacy with immune activation symptoms of fever, vascular leak and shock. Interferon alfa-2a/b (IFN-α) is also active in many cancers, including melanoma, renal cell carcinoma, Kaposi’s sarcoma and hematologic malignancies (20). IFN-α activates NK cells, increases MHC class I expression, and promotes a Th1 immune profile, all of which can drive an anti-tumor immune response. Systemic toxicity is also limiting for IFN-α therapy, with fever, fatigue, malaise and cytopenias being most common. Many studies have evaluated cytokine therapy in the treatment of MPM. Intravenous, subcutaneous and intrapleural administration of IL-2 has shown some effects on tumor regression in MPM (21,22). IL-2 may exert effects through promotion of tumor-infiltrating lymphocytes (TILs), as well as reduction in the micro-vessel count (23). Subcutaneous IFN-α-2a was found to be somewhat efficacious and reasonably well-tolerated, with a 14% overall response rate as monotherapy for MPM (24). Cytokine gene therapy has been explored as a modality to deliver high local doses of cytokines while limiting systemic toxicities. In a recent study, intrapleural therapy with a modified adenovirus containing the human IFN-α-2b gene (Ad.IFN) was given in combination with systemic chemotherapy. In the cohort of previously treated patients, second-line therapy with chemotherapy and Ad.IFN demonstrated a median overall survival of 21.5 months, which was numerically better than historical reports of second-line therapy (25).
In the modern era, immunotherapy has been reinvigorated by the use immune checkpoint inhibitors (CPI) targeting CTLA-4 and the PD-1/PD-L1 pathways (26). The first commercially-available CPI was ipilimumab, a CTLA-4 antibody which blocks inhibitory signaling mediated by CTLA-4 on the immune effector T-cell. Ipilimumab has been shown to be an effective treatment for advanced melanoma in both previously-treated and treatment-naive patients (27,28), and also as adjuvant therapy for resected melanoma with lymph node involvement (29). Inhibition of CTLA-4 has also been explored as therapy for MPM with disappointing results. An initial single-arm, phase II study of the CTLA-4 antibody tremelimumab showed a disease control rate of 38% in previously-treated MPM (30). A subsequent randomized phase III study of tremelimumab vs. placebo in previously-treated MPM was recently completed. This study of 571 patients showed no significant improvement in overall survival for tremelimumab vs. placebo, 7.7 vs. 7.3 months, respectively (31).
Antibodies targeting PD-1 and PD-L1 have demonstrated remarkable activity in a variety of malignancies. Commercially available agents include pembrolizumab, nivolumab, and atezolizumab. The PD-1 antibody pembrolizumab is currently FDA-approved for treatment of melanoma, PD-L1+ non-small cell lung cancer, and platinum-treated head and neck squamous cell cancers. Pembrolizumab has also demonstrated efficacy in the treatment of MPM. In a phase I study of 25 patients with PD-L1+ MPM, pembrolizumab demonstrated an overall response rate of 20%, with an additional 52% showing stable disease for at least 6 months; the median overall survival was 18.0 months, and four patients had ongoing response at nearly 2 years from initiation of therapy (32). The PD-1 antibody nivolumab is also being investigated in MPM. Preliminary data from a phase II study demonstrated a disease control rate of 50% at 12 weeks with 5 of 34 evaluable patients showing a partial radiographic response (33). The PD-L1 antibody avelumab has also been investigated in MPM. In a phase Ib study of 53 previously-treated patients with unresectable pleural or peritoneal mesothelioma, the unconfirmed objective response rate was 9.4% (all partial responses), median progression-free survival was 17.1 weeks, and toxicities were manageable (34).
The available data confirm immunotherapy as a viable treatment modality for MPM. Despite the remarkable durability of responses seen in some patients, the overall response rates with immunotherapy remain low at 10–20%. Future studies will be evaluating combination approaches to enhance the activity of immunotherapy agents. Combination treatments with CTLA-4 and PD-1 antibodies have already demonstrated improved efficacy in melanoma therapy, albeit with increased toxicity (35). Combination therapy with nivolumab and ipilimumab is being investigated in previously-treated MPM (36) as well as first-line therapy compared to standard chemotherapy in treatment-naive patients (37). Anti-angiogenic agents may improve trafficking of TILs into the tumor microenvironment. The use of anti-angiogenic therapy with bevacizumab and the PD-L1 antibody atezolizumab is being evaluated in rare tumors, including MPM (38).
Combining radiation therapy and immunotherapy for mesothelioma
Radiation therapy has long been used in the treatment of MPM. Most commonly, radiation therapy has been used for palliation of symptoms or to prevent impending symptoms in patients with advanced disease. Radiation therapy also has an established role as adjuvant therapy in patients undergoing surgical resection. Less commonly, radiation therapy may be considered in select cases as a definitive therapy in patients who are not surgical candidates or who are experiencing isolated or more limited progression on or after system therapy.
With the increasing recognition of the importance of systemic immunotherapy across numerous disease sites, including mesothelioma, it is also notable to recognize that radiation therapy may itself be immunomodulatory. Radiotherapy has been shown to upregulate TILs (39). Radiation therapy can also activate CD8 T-cells and enhances MHC class I expression that allows for the immune system to react to tumor neoantigens (40,41). Furthermore, it has been recognized that delivery of radiation therapy to a local tumor site in select cases can simultaneously achieve radiation-induced cell death locally and a tumor response outside of the radiation portal. This out-of-field tumor response, thought to be the result of a stimulatory signal to the immune system from the local anti-tumor effects of irradiation, is termed “abscopal” effect, meaning “away from the target” (42).
Research in other disease sites, including that of melanoma (43,44), suggests that the immunomodulatory properties of radiation therapy, as assessed either by CD8 activation and the ability to induce an abscopal response or by the upregulation of regulatory T cells, may be greater for hypofractionated treatment courses when compared with conventional fractionation. However, other murine studies suggest potential benefits of conventional fractionation for immunomodulation (45). Clinical data to support one approach or the other in MPM is, at this time, lacking.
While knowledge that irradiation can induce an abscopal effect has long been known (40), interest in the use of radiation therapy as a means to induce an immune response has been dramatically intensified in the past decade due to the proliferation of cancer-directed immunotherapies (46). Radiation therapy can achieve an abscopal effect with or without immunotherapy. The combination of radiation therapy and immunotherapy can serve to achieve a synergistic therapeutic effect (47). Specifically, irradiation can increase tumor antigen production and presentation. Given the increasing evidence demonstrating a potential role for CPI for mesothelioma, radiotherapy has also been shown to enhance the antitumor immune responses of CPI (48). Radiation therapy can achieve synergy with CPI by upregulating cytotoxic T-lymphocyte activity (47) and downregulating myeloid-derived suppressor cells (49).
While the optimal dose-fractionation regimen of radiation therapy to induce an abscopal response has not been determined, a prominent line of thinking is that stereotactic body radiation therapy (SBRT), also termed stereotactic ablative radiation therapy (SABR) (50,51), may be the radiation therapy modality that holds the most promise when delivered in combination with immunotherapy. By delivering high, ablative doses of irradiation, SBRT induces a robust immune response that can allow for the upregulation of cellular expression of major histocompatibility complex I, immunomodulatory cytokines, inflammatory mediators, heat shock proteins, death receptors, costimulatory molecules, and adhesion molecules, all of which can augment the antitumor immune responses of immunotherapy (52). Additionally, due to the immunologic effects of proton therapy and its ability generally to better spare critical normal structures from excessive irradiation in the thorax, proton therapy may also hold promise as an optimal radiation therapy modality to be used in combination with immunotherapy for MPM (53-55).
The data on combining radiation therapy and immunotherapy in MPM to date is exceedingly spare. A recent report by investigators at University of Toronto found that the growth of primary tumors in a murine mesothelioma model was significantly inhibited by localized radiation therapy, and that CTLA-4 antibody enhanced the antitumor effect (56). Interestingly, they found that the growth of a second tumor not treated with localized radiotherapy was delayed when the primary tumor was radiated. In their analysis, localized irradiation increased T cell infiltration (for both Treg and cytotoxic T cells) into both the irradiated and un-irradiated tumors, and that the proportion of Treg to effector T cells in both tumors was reversed after blockade with CTLA-4, whereas CD8 T cells upregulated. With an increase in immune-related gene expression, radiotherapy was found to increase of tumor-infiltrating T cells, and CTLA-4 blockade allowed for reductions of Tregs and increases in cytotoxic T cells. Their findings suggest that an abscopal effect is feasible to be achieved in mesothelioma, and that this effect from radiotherapy can be enhanced with the modulation of the T cell immune response that can be achieved with immune CPI.
Reported clinical data on combining immune CPI and radiation therapy, to date, are lacking. While awaiting clinical outcomes and toxicity data, practitioners should be aware of the potential for enhanced pulmonary toxicity when combining radiation therapy and immunotherapy. Among the potential toxicities of thoracic radiotherapy, patients are at risk for acute or subacute radiation pneumonitis that can occur in the weeks to months following treatment completion. Radiation pneumonitis is an inflammation of the lung tissues from irradiation that results in hyperemia, increase capillary permeability, cytokine release, leukocytic infiltration, and exudative alveolitis (57). Radiation pneumonitis can be highly symptomatic, with patients commonly presenting with dyspnea, nonproductive cough, low grade fevers, and pleuritic chest pain, and in some cases symptoms patients can develop respiratory compromise that can be life threatening (58).
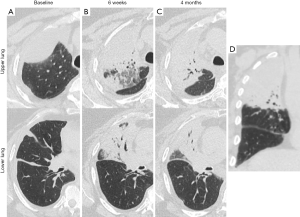
The acute and subacute lung changes from radiation pneumonitis can lead to chronic parenchymal damage and the development of late fibrosis (59). Although less commonly experienced, patients receiving immune CPI are also at risk of developing pneumonitis even in the absence of radiotherapy (60). At this time, it is unclear if the incidence of pneumonitis from radiation therapy to the thorax is higher if delivered concurrently or in close proximity to immune CPI and if pneumonitis symptoms would be more severe with the treatment combination in patients who do develop pneumonitis (Figure 1). As such, until more clinical data are available, providers should be vigilant about monitoring for symptoms or signs of pneumonitis in patients receiving thoracic radiation therapy with immunotherapy. Further mechanistic insights gained from preclinical studies and analysis of clinical samples may help guide rational therapeutic strategies designed to mitigate toxicity.
Conclusions
The recent studies demonstrating the efficacy of immunotherapy in the treatment of MPM have fueled interest in the immunomodulatory properties of radiation therapy in this disease. Combination treatments with immunotherapy and radiation therapy could potentiate antitumor effects by inducing an immunogenic cell death that promotes a robust T-cell response and favorable microenvironment for tumor antigen presentation and systemic immune response. Ongoing clinical trials will help define the role of radiation and immunotherapy in the treatment of mesothelioma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1:491-96. [PubMed]
- Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol 2012;227:44-58. [Crossref] [PubMed]
- Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022-5. [Crossref] [PubMed]
- Inai K. Pathology of mesothelioma. Environ Health Prev Med 2008;13:60-4. [Crossref] [PubMed]
- Goudar RK. Review of pemetrexed in combination with cisplatin for the treatment of malignant pleural mesothelioma. Ther Clin Risk Manag 2008;4:205-11. [Crossref] [PubMed]
- Wolf AS, Flores RM. Current Treatment of Mesothelioma: Extrapleural Pneumonectomy Versus Pleurectomy/Decortication. Thorac Surg Clin 2016;26:359-75. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. MARS trialists. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Friedberg JS, Culligan MJ, Mick R, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg 2012;93:1658-65. [Crossref] [PubMed]
- Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 2009;63:94-7. [Crossref] [PubMed]
- Ceresoli GL, Zucali PA, De Vincenzo F, et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer 2011;72:73-7. [Crossref] [PubMed]
- Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology 2007;121:1-14. [Crossref] [PubMed]
- Richardson MA, Ramirez T, Russell NC, et al. Coley toxins immunotherapy: a retrospective review. Altern Ther Health Med 1999;5:42-7. [PubMed]
- Kumar T, Patel N, Talwar A. Spontaneous regression of thoracic malignancies. Respir Med 2010;104:1543-50. [Crossref] [PubMed]
- Floros T, Tarhini AA. Anticancer Cytokines: Biology and Clinical Effects of Interferon-α2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin Oncol 2015;42:539-48. [Crossref] [PubMed]
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451-8. [Crossref] [PubMed]
- Ferrantini M, Capone I, Belardelli F. Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie 2007;89:884-93. [Crossref] [PubMed]
- Castagneto B, Zai S, Mutti L, et al. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: Results of a phase II study on 31 consecutive patients. Lung Cancer 2001;31:303-10. [Crossref] [PubMed]
- Lucchi M, Chella A, Melfi F, et al. Four-modality therapy in malignant pleural mesothelioma: a phase II study. J Thorac Oncol 2007;2:237-42. [Crossref] [PubMed]
- Alì G, Boldrini L, Lucchi M, et al. Treatment with interleukin-2 in malignant pleural mesothelioma: immunological and angiogenetic assessment and prognostic impact. Br J Cancer 2009;101:1869-75. [Crossref] [PubMed]
- Robinson BW, Manning LS, Bowman RV, et al. The scientific basis for the immunotherapy of human malignant mesothelioma. Eur Respir Rev 1993;3:195-8.
- Sterman DH, Alley E, Stevenson JP, et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNα Combined with Chemotherapy. Clin Cancer Res 2016;22:3791-800. [Crossref] [PubMed]
- Ribas A. Releasing the Brakes on Cancer Immunotherapy. N Engl J Med 2015;373:1490-2. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522-30. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015;3:301-9. [Crossref] [PubMed]
- Kindler HL, Scherpereel A, Calabrò L, et al. Tremelimumab as second- or third-line treatment of unresectable malignant mesothelioma (MM): Results from the global, double-blind, placebo-controlled DETERMINE study. J Clin Oncol 2016;34;abstr 8502.
- Alley EW, Lopez L, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Quispel-Janssen J, Zago G, Schouten R, et al. OA13.01 A Phase II Study of Nivolumab in Malignant Pleural Mesothelioma (NivoMes): with Translational Research (TR) Biopsies. J Thorac Oncol 2017;12:S292-S293. [Crossref]
- Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase 1b trial: safety, clinical activity and PD-L1 expression. J Clin Oncol 2016;34;abstr 8503.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Ipilimumab and Nivolumab in the Treatment of Malignant Pleural Mesothelioma: a Phase II Study (INITIATE). Available online: https://clinicaltrials.gov/ct2/show/NCT03048474
- Study of Nivolumab Combined With Ipilimumab Versus Pemetrexed and Cisplatin or Carboplatin as First Line Therapy in Unresectable Pleural Mesothelioma Patients (CheckMate743). Available online: https://clinicaltrials.gov/ct2/show/NCT02899299?term=A+Phase+III%2C+Randomized%2C+Open+Label+Trial+of+Nivolumab+in+Combination+With+Ipilimumab+Versus+Pemetrexed+With+Cisplatin+or+Carboplatin+as+First+Line+Therapy+in+Unresectable+Pleural+Mesothelioma&rank=1
- Atezolizumab and Bevacizumab in Rare Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03074513
- Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005;174:7516-23. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. [Crossref] [PubMed]
- Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [Crossref] [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. [Crossref] [PubMed]
- Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589-602. [Crossref] [PubMed]
- Simone CB 2nd, Burri SH, Heinzerling JH. Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies. Transl Lung Cancer Res 2015;4:545-52. [PubMed]
- Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831-8. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Simone CB 2nd, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784-90. [Crossref] [PubMed]
- Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer 2017;123:688-96. [Crossref] [PubMed]
- Finkelstein SE, Timmerman R, McBride WH, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol 2011;2011:439752. [Crossref] [PubMed]
- Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;95:505-16. [Crossref] [PubMed]
- Simone CB 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J 2014;20:427-32. [Crossref] [PubMed]
- Wink KC, Roelofs E, Solberg T, et al. Particle therapy for non-small cell lung tumors: where do we stand? A systematic review of the literature. Front Oncol 2014;4:292. [Crossref] [PubMed]
- Wu L, Wu MO, De la Maza L, et al. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015;6:12468-80. [Crossref] [PubMed]
- Ding NH, Li JJ, Sun L. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets 2013;14:1347-56. [Crossref] [PubMed]
- Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environ Health Perspect 1986;70:261-91. [Crossref] [PubMed]
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281-93. [Crossref] [PubMed]
- Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One 2016;11:e0160221. [Crossref] [PubMed]


