Special topics in immunotherapy and radiation therapy: reirradiation and palliation
Immunotherapy has revolutionized the treatment of non-small cell lung cancer (NSCLC). In advanced disease, inhibitors of the PD-1/PD-L1 pathway have proven superior to cytotoxic chemotherapy in all-comers in the second-line setting (1-3), and in patients with high expression of PD-L1 in the first-line setting (4). Also showing promise in lung cancer are CTLA-4 blocking antibodies (5). Not yet established is the proper way to incorporate these agents in earlier stages of NSCLC, nor do we completely understand how these agents can be used safely in conjunction with radiation. There is, nevertheless, evidence that radiation can enhance the efficacy of immune agents even outside of the radiation portal. This review focuses on what is known about the use of immunotherapy in concert with palliative radiation as well as what opportunities exist of the potential benefit of immunotherapy to assist in salvaging patients eligible for definitive re-irradiation.
Palliative radiation and immunotherapy
Palliative radiation is a cornerstone of the treatment for patients with advanced, metastatic NSCLC. While this is clearly a systemic disease requiring a systemic approach, individual tumors causing local complications can negatively impact a patient’s quality of life, and radiation may ameliorate many such local complications. Patients often require relief more quickly and reliably than our systemic treatments can provide. In addition, for central nervous system (CNS) disease, systemic approaches are less likely to be effective due to the blood brain barrier. Therefore, often in the treatment of metastatic NSCLC, there exists simultaneously a need to initiate radiation treatment along with a desire to embark on a proven-effective systemic therapy without additional delay. Can radiation and immunotherapy be delivered together safely?
The toxicities of immunotherapy are, logically, immune-mediated (immune-related adverse events or irAE’s). Generally, irAE’s have been more severe with the anti-CTLA-4 monoclonal antibodies such as ipilimumab which is approved for the treatment of melanoma. The PD1/PD-L1 inhibitors such as the three currently approved for use in NSCLC (nivolumab, pembrolizumab, and atezolizumab) are generally less toxic. However, combinations of CTLA-4 and PD-1/PD-L1 agents are being actively explored in clinical trials in NSCLC. While adverse events related to immunotherapies are generally less common than what we are used to seeing with traditional cytotoxic chemotherapy, the most commonly observed toxicities include rash, thyroid and pituitary dysfunction, colitis, nephritis, hepatitis, and pneumonitis. Fatal cases of pneumonitis have occurred, and given this is an overlapping toxicity of radiation therapy to the chest, it represents a particular concern.
The pivotal randomized studies leading to the approval of the checkpoint inhibitors in NSCLC included strict instructions about the use of radiation. They also excluded patients with active brain metastases. In the study of first-line pembrolizumab vs. chemotherapy (Keynote 24), patients were ineligible if they had received thoracic radiation >30 Gy within 6 months of the first dose of study drug (6). In the second-line nivolumab studies, prior radiation or surgery must have been completed at least two weeks prior to randomization (1,2), and patients with interstitial lung disease were also excluded. Therefore, in the large prospective studies, radiotherapy was limited due to the concern for pneumonitis.
Pneumonitis risk of immunotherapy with thoracic radiation
Pneumonitis is one of the most feared complications of immunotherapy and early on was known to be a potential cause of death in treated patients (7). In a detailed retrospective review of 915 patients treated with anti-PD-1/PD-L1 monoclonal antibodies, 43 patients (5%) developed pneumonitis and 1% developed grade 3 or higher (8). Two hundred nine of the patients had NSCLC of which nine had pneumonitis. Sixty-one percent of the patients with pneumonitis had a RECIST response. The incidence of pneumonitis was higher in patients receiving combination immunotherapy (PD-1/PD-L1 inhibitor given in conjunction with anti-CTLA-4 mAb) versus those receiving single agent (10% vs. 3%, P<0.01), but there was no difference in pneumonitis rates between patients receiving PD-1 vs. PD-L1 inhibitors. The incidence was similar in patients with melanoma vs. NSCLC. The majority of patients developing pneumonitis [27/43 (63%)] had not received prior thoracic radiation, and pneumonitis was seen in both never smokers [19 of 43 (44%)] and current/former smokers [24 of 43 (56%)]. The median time to pneumonitis onset was 2.8 months (range, 9 days to 19.2 months) and occurred earlier in patients getting combination therapy (median 2.7 months, range, 9 days to 6.9 months) than monotherapy (4.6 months, range, 21 days to 19.2 months). The grade of pneumonitis was grade 1 in 40%, grade 2 in 33%, 23% grade 3, and one patient each (2%) experienced grade 4 and 5 toxicities. Dyspnea (53%), cough (35%), fever (12%) and chest pain (7%) were the most common presenting symptoms. The majority of grade 1/2 patients were managed as outpatients while 19% of the grade 2 and all of the higher grade patients were hospitalized. Treatment consisted of holding drug (which was the only approach in 88% of the grade 1 patients) and initiating corticosteroids (all patients with grade 2 or higher toxicity and 12% of the grade 1 patients). Five of 12 grade 3 patients received additional immunosuppression (infliximab in three and two with both infliximab and cyclophosphamide). The median starting dose of prednisone was 50 mg (range, 20 to 80 mg) and the median duration of corticosteroid treatment was 68 days (range, 20 to 154 days). Pneumonitis improved and resolved in 88% of patients. Five patients clinically worsened while on treatment and ultimately died, one of the pneumonitis itself, three due to infections related to immunosuppression, and one of progressive malignancy. These were the same five patients who received immunosuppression in addition to steroids. Twelve patients were rechallenged with immunotherapy following a complete resolution of their pneumonitis. Nine patients did not get recurrent pneumonitis, and of the three (25%) who did, one resolved with holding drug only and two others with corticosteroids.
In the above mentioned review of pneumonitis from immunotherapy, chest X-rays were not always diagnostic, showing possible pneumonitis in only six of nine cases. One X-ray was interpreted as showing progressive cancer and two had no reported new radiographic abnormality. Radiographic features of pneumonitis consisted of cryptogenic organizing pneumonia-like in 19%, ground glass opacities in 37%, interstitial (7%), hypersensitivity (22%) and not otherwise specified (15%). Eleven patients underwent biopsy at time of pneumonitis diagnosis. Histopathologic findings included cellular interstitial pneumonitis (4/11), organizing pneumonia (3/11), diffuse alveolar damage (1/11), and no observed abnormalities (3/11). Of the five patients who did not improve, all were current smokers (P=0.053 vs. not current smokers) and four of them had underlying lung conditions (P=0.047).
In another series presented at ASCO 2016, 24 patients with NSCLC and pneumonitis from PD-1 or PD-L1 inhibitors were identified (9). Five had been treated in combination with an anti-CTLA4-Ab and one in combination with chemotherapy. The response rate across all patients was 50%. The median onset to pneumonitis was 75 days (range, 8–549 days). Nineteen patients were treated with steroids. All resolved to grade 0 or 1. Three types of radiographic patterns were noted: organizing pneumonia (consolidation with or without ground glass opacity in peripheral/peribronchovascular distribution) (52%), ground glass opacity (diffuse or focal GGO’s) (28%), and nodular (perilymphatic or centrilobular nodules) (8%), while 12% had a mixed pattern. Transbronchial biopsies were done on 20 patients. All biopsied patients were found to have varying degrees of lymphocytic infiltrate, mainly T cells. Seven also had granulomas, and eight had eosinophils. CD8 cells predominated with a CD4/CD8 ratio of 0.46.
Of course, pulmonary toxicity in the form of radiation pneumonitis and radiation fibrosis are well-recognized dose limiting toxicities (DLTs) and common adverse events associated with radiation to the lung. Therefore, the combination of immunotherapy with radiation is a potential concern. The likelihood of radiation pulmonary toxicity is dependent on dose. Therefore, this is a greater concern for patients undergoing definitive radiation. Radiation pneumonitis generally occurs approximately 12 weeks following pulmonary irradiation and involves the accumulation of inflammatory cells within the interstitium (10). Concurrent traditional cytotoxic chemotherapy clearly increases the risk of pneumonitis. Risk factors for pneumonitis from radiation include treatment factors such as use of concurrent chemotherapy, radiation dose, volume of lung irradiated, and fractionation schedule. Also important are patient factors like poor pulmonary function, smoking status, and pre-existing lung disease.
Is it safe to administer palliative radiation to the lung simultaneous with currently approved immunotherapy? Unfortunately, data are limited. However, in a phase I study of ipilimumab in conjunction with stereotactic ablative radiation (SABR) therapy in patients with metastatic solid tumors, fourteen patients had lung lesions radiated (11). None of the patients developed > grade 1 pneumonitis. However, one patient with a significant metastatic disease burden and a recent myocardial infarction died following the second ipilimumab dose and after two out of four planned fractions of lung SABR; cause of death was unknown, and the patient was not experiencing any treatment-related toxicity at the time of death. In another phase I study of patients with metastatic melanoma (12), patients received ipilimumab 3–5 days following SABR to an index lesion. In the 22 patients treated of whom 10 had lung lesions radiated (either 8 Gy × 2 or 8 Gy × 3), there were no DLTs observed. DLTs were defined as any treatment-related grade 4 or higher immune related toxicity during the study or within 30 days of the last dose of ipilimumab. These studies as well as other ongoing studies addressed below will hopefully provide better answers in the near future.
Radiation of brain metastases in patients on immunotherapy
Registration trials of immune therapies excluded patients with active brain metastases and thus far there have been no published data evaluating the pharmacokinetic or pharmacodynamic data within brain tissue to evaluate penetration into brain tissue or brain tumors (13). The treatment of brain metastases with systemic agents has been challenging due to the blood-brain barrier.
Theories as to how immune-oncology agents may treat brain tumors include disruption of the blood brain barrier (made up of endothelial cells, astrocytes, and pericytes) by the brain metastases themselves allowing for migration of agents into the perivascular space. This phenomenon is also seen with many chemotherapy agents that do not have much CNS penetration under normal circumstances, but nevertheless can lead to tumor response within the CNS (14). Another possibility is not that the drug itself enters the CNS, but that the T cells are activated peripherally and then are able to enter the CNS. Preclinical data in animals with brain tumors has shown improved survival for those treated with CTLA-4 and PD-1 blockade as well as with the combination (15-17).
Immunotherapy was initially approved in melanoma based upon a three-arm randomized trial in which the two ipilimumab arms (ipilumimab alone and ipilimumab with an experimental vaccine) demonstrated improved survival compared to the vaccine alone (18). Because of this and the propensity of melanoma to spread to the brain, much of the data for use of radiation to the brain in combination with immunotherapy has been in melanoma. Multiple studies have demonstrated the efficacy of ipilimumab in the treatment of brain metastases from melanoma with response and disease control rates similar (range, 19–55%) to those seen systemically, suggesting the blood-brain barrier is actually not much of a “barrier” (19-21). In a study of pembrolizumab in patients with untreated brain metastases from either NSCLC or melanoma, 6 of 18 (33%) of the patients with lung cancer and four out of 18 (22%) of the patients with melanoma experienced responses in the brain (22).
Nevertheless, these results are not as good as what are typically seen with stereotactic radiosurgery (SRS) for the treatment of brain metastases (23), and progressive disease within the brain can cause significant morbidity and mortality (19). Therefore, the combination of radiation therapy and immunotherapy may be required for more reliable durable control. In a series of 77 patients with metastatic melanoma with brain metastases all of whom were treated with SRS to the brain and 27 additionally received ipilimumab, the ipilimumab group had a 21.3 months median survival vs. 4.9 months (24). In another study of 25 patients with melanoma brain metastases treated with SRS and ipilimumab vs. 33 treated with SRS alone, no benefit in overall survival (OS) was observed (25). A third study also showed a survival advantage for those patients undergoing ipilimumab in addition to SRS for brain metastases, though there were prognostic imbalances in the study favoring the immunotherapy arm (26). Symptomatic radiation necrosis has been noted in patients treated with a combination of immunotherapy and SRS. Case series have suggested a connection (27,28), though in randomized comparisons it is not clear that the rates are any higher than with SRS alone.
The abscopal effect for palliation
One attractive potential advantage of delivering palliative radiation to patients with metastatic disease is the hope of eliciting an abscopal effect. This means causing a response in tumors distant from the radiated lesion (Ab-scopus is Latin for “away from the target”). Radiation therapy may serve to act as an in situ vaccine. Release of tumor antigens during radiation therapy may prime the immune system to act on disease outside the radiation portal, leading to the abscopal effect (29). This rare phenomenon has been observed prior to the advent of immunotherapy. Patients who have previously progressed on immunotherapy have been shown to have a systemic response following administration of local radiation (30). In mouse models, the abscopal effect is only observed in the presence of a functioning immune system (31,32). There are many theories as to why radiation would lead to such a phenomenon. Radiation induces upregulation of immunogenic cell surface markers (33-35). Radiation-induced inflammation can cause secretion of cytokines as well as infiltration of tumor-specific T cells through vascular normalization (36,37). Radiation can also lead to the destruction of potentially immunosuppressive stromal cells. It may also lead to improved T cell extravasation and homing to tumors (37) and radiation therapy can directly upregulate the PD-1/PD-L1 pathway (38). Case reports exist where it appears that immunotherapy can enhance an abscopal effect when radiation is delivered along with it (30).
Ongoing studies in palliative radiation therapy and immunotherapy
Table 1 depicts studies that are anticipated or currently open which combine radiation therapy and immunotherapy in lung cancer. An exploration of these studies demonstrates that a variety of radiation regimens and immunotherapy agents are recommended, and that the endpoints range from safety to response rate to survival outcomes such as PFS. It is also clear given the large number of trials that within the next 5–10 years, much more will be known about the combination of these two modalities, including in the context of palliative radiation. As one example, a recent study examined the safety, efficacy, and immunologic correlates from peripheral T cells with the combination of ipilimumab and SABR to the liver or lung. Of the 35 patients in this trial, the authors found that the rate of grade 3 or higher toxicity was 40%. Approximately 25% of patients experienced what was defined as a “clinical benefit”, a partial response or stable disease at least 6 months in duration. Several T cell markers were found to be associated with a clinical benefit, including increases in CD8+ cells, CD8+/CD4+ T-cell ratio, and the proportion of CD8+ T cells that expressed 4-1BB and PD1 (11). The study suggests that toxicity with this combination regimen needs to be closely monitored, and therefore that much data is needed regarding the frequency and relevance of the abscopal effect with this paradigm.
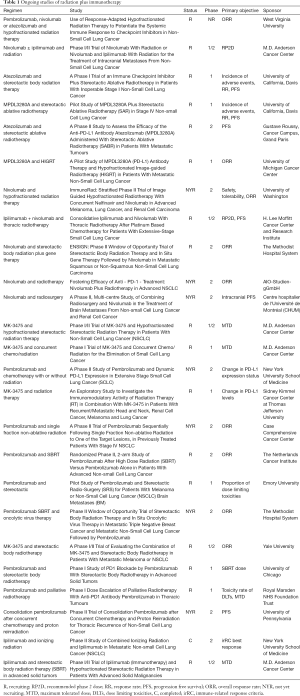
Full table
R, recruiting; RP2D, recommended phase 2 dose; RR, response rate; PFS, progression free survival; ORR, overall response rate; NYR, not yet recruiting; MTD, maximum tolerated dose; DLTs, dose limiting toxicities, C, completed; irRC, immune-related response criteria.
Reirradiation and immunotherapy
Locoregional recurrence (LRR) remains a leading cause of death in lung cancer (39,40). Therefore, the primary option in this clinical context has historically been systemic therapy, but response rates for salvage chemotherapy are typically low (41,42). Consequently, due in part to technologic advancements in radiation therapy, in the past several years, there has been increasing interest in reirradiation. There are two primary scenarios that pertain to reirradiation in the setting of locoregionally recurrent diseases (LRRs). The first is in the context of lung parenchymal recurrences, in which SABR is an option. The second pertains to LRRs involving critical structures such as the mediastinum, in which conventionally fractionated radiation (± chemotherapy) are the only feasible option if the intent is definitive.
SABR as reirradiation for parenchymal recurrence
There have been several reports of SABR alone for this indication. Overall, these trials have shown that treatment is tolerated well and with promising short-term control rates. For example, in one study of 278 patients, 26 (29 tumors) were treated with SABR for recurrent disease. One- and 2-year progression-free survival rates were 56% and 37%, respectively, with no grade 3 or higher toxicities (43). In a publication from Memorial Sloan-Kettering Cancer Center, 39 patients previously treated with conventionally fractionated radiation were then retreated with SABR for either recurrent primary or metastatic disease. Median recurrence-free survival and OS rates were 13.8 and 22.0 months, respectively, and patients without overlap in the high dose regions had both higher biologically effective doses as well as better control rates. In this study, grade 3 toxicity was approximately 20% (44). Finally, a report from MD Anderson Cancer Center assessing the role of SABR and measuring the rate of toxicity and control in centrally/superiorly located or isolated recurrent NSCLC showed that, while in-field control rate was 100%, 20% of patients developed mediastinal nodal recurrence and an additional 35% eventually had distant metastases. Of note, one patient with an apical tumor did develop brachial plexopathy, and 11% of other patients had grade 2–3 chest wall pain (45).
Standard fractionated reirradiation for LRR
There are also several reports of reirradiation outside of the context of SABR (46-52), but most are limited by their retrospective nature, small patient numbers, and heterogeneity in diagnosis, treatment intent, and radiation dose/fractionation regimens. In particular, reports of definitive locoregional radiation for curative intent using advanced techniques in the reirradiation setting are sparse, and a brief examination of the literature demonstrates these limitations. A recent study from Japan examined 21 patients treated with both NSCLC and SCLC who had LRR and an overlap of prior dose distributions at the 80% dose level. The median time from initial course to reirradiation was 26.8 months, and the median dose of reirradiation was 60 Gy in 2 Gy equivalents. The authors reported median local PFS to be 12.9 months, with a median OS time of 31.9 months. There was only one grade 3 or higher complication, radiation pneumonitis, leading the authors to conclude that the regimen appeared safe. Interestingly, patients treated to a dose of ≥60 Gy had improved local PFS (53). In another study, 24 patients were identified who received standard fractionated therapy both for the initial disease and at the time of LRR. The median dose at initial treatment was 59.8 Gy, and at reirradiation was 60 Gy in 2 Gy fractions. The median interval between courses of radiation was 51 months, and median OS after reirradiation was 13.5 months. The median survival at 1 year was 51%. Toxicity was non-negligible, with 3/24 patients possibly experiencing a grade 5 adverse event (bleeding) (54).
Investigators from MD Anderson Cancer Center recently published the largest series of retrospective and prospectively analyzed patients. In this study, 102 patients underwent reirradiation with intensity modulated radiation therapy (IMRT) or proton beam therapy (PBT) for intrathoracic recurrent NSCLC and were not candidates for SABR. The authors found that median local failure-free survival (LFFS), distant metastasis-free survival (DMFS), and OS times were 11.4 months (range, 8.6–22.6 months), 11.4 months (range, 6.3–23.8 months), and 14.7 months (range, 10.3–20.6 months), respectively. Notably, of the patients who developed local failure after reirradiation, 88% occurred within the original or reirradiation field. Squamous cell cancer (SCC) histology and T4 disease at the time of reirradiation were associated with reduced local control. Toxicity was acceptable, with a <10% rate of grade ≥3 toxicity. The authors concluded that IMRT and PBT appeared to be feasible options for treating recurrent NSCLC, though local failure and distant metastasis were still common, and that the overall benefit compared to systemic therapy was questionable. Furthermore, patients in which at least 6 months had elapsed since the initial course of radiation had superior survival outcomes. Finally, the authors concluded that randomized trials are needed to more definitively address the role of reirradiation in this scenario (47). Figure 1 depicts the isodose curves from a patient that is reported in this study, with both the initial course and reirradiation course. Note that in the reirradiation setting proton therapy was utilized to achieve maximum dose falloff given the proximity of several critical structures, and that with this approach the esophagus was entirely spared in the reirradiation plan.
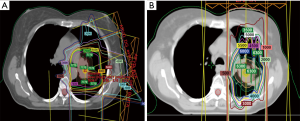
Proton reirradiation for LRR
The University of Pennsylvania recently published data from a prospective trial studying the potential toxicities of proton reirradiation for local recurrences of NSCLC (55). Proton therapy has been utilized as a potential strategy to minimize harm to surrounding tissues while treating thoracic tumors aggressively. Therefore proton therapy is ideally suited to minimize toxicities in the reirradiation setting. This multi-institutional trial included 57 patients with recurrent NSCLC in or near their prior radiation field. Ninety-three percent of patients completed the reirradiation course. Sixty-seven percent received concurrent chemotherapy. Patients with high tumor volume (clinical target volume-to-internal target volume ratio ≥250 cm3) were closed to enrollment owing to infeasibility. The 1-year rates of overall and progression-free survival were 59% and 58%, respectively. In total, grade 3 or higher acute and/or late toxicity developed in 24 patients (42%), acute toxicity developed in 22 (39%), and late toxicity developed in seven (12%). Six grade 5 toxicities were observed. Increased overlap with the central airway region, mean esophagus and heart doses, and concurrent chemotherapy were associated with significantly higher rates of grade 3 or higher toxicity. Decreased OS was seen with increased mean esophagus dose (P=0.007). While proton reirradiation was deemed feasible in the low tumor volume setting, given the observed toxicities, the authors cautioned providers to consider tumor volume, location, and relevant dosimetric parameters when selecting patients for treatment with this modality.
Conclusions of reirradiation and proposed algorithm for evaluation
The primary conclusions that can be drawn by the current reirradiation literature in thoracic malignancies are as follows. First, while in principal there may be a benefit to targeting recurrent localized disease with radiation therapy, the evidence supporting reirradiation in this setting is evolving, without strong comparative effectiveness data demonstrating superiority of reirradiation compared to second-line systemic therapy. Second, reirradiation in the context of parenchymal recurrences is stronger than that of standard fractionated radiation with mediastinal relapse, with retrospective trials demonstrating higher control rates and lower toxicity with stereotactic treatment when feasible (e.g., without exceeding critical structure constraints at hypofractionated doses).
Figure 2 presents one algorithm that can be used in approaching a patient with LRR who has previously received high dose radiation. Pertinent decision points are the feasibility of SABR, the role of chemotherapy in conjunction with radiation, and patient/disease characteristics that would preclude definitive treatment given the high potential toxicity, including performance status, time since initial course of radiation (with 6 months being a reasonable cutoff as noted above), and the absence or presence of distant metastases. Of note, many patients may be candidates for a palliative course of reirradiation rather than a definitive course, and the regimen of 8 Gy × 1 has been shown to provide “safe, effective, and durable pain palliation” for patients who had previously received concurrent chemoradiation to a total dose of 52–66 Gy (56). Three additional guiding principles can also be used in this context. First, if a surgical option is present, it should strongly be considered. Second, when there is overlap with prior radiation fields, patients should be consented to high-grade and even fatal toxicity such as radiation pneumonitis, esophageal fistula, bronchial stricture/fistula, and grade 5 bleeding. Finally, the best context for reirradiation is in a clinical trial when available.

The role of immunotherapy and reirradiation clinical trials
There is a clear role for immunotherapy to enhance the efficacy of reirradiation. As systemic therapies improve, the observed incidence of isolated LRR is likely to continue to increase. Furthermore, patients treated with reirradiation appear to have a higher potential for both local recurrence (due to established radioresistance) as well as systemic failure (due to aggressive disease), and the addition of immunotherapy could potentially improve both of these endpoints. In an ideal scenario, high dose reirradiation would reduce the rate of local progression, while the abscopal effect of combined modality treatment would improve the rate distant metastases. The University of Pennsylvania opened a phase II study of consolidation pembrolizumab after concurrent chemotherapy and proton reirradiation for thoracic recurrences of NSCLC. There are several unanswered questions. First, will the risk of toxicities such as pneumonitis and cardiac toxicity (5,57) with both checkpoint inhibitors and radiation preclude combination of these agents? Second, what is the optimal timing of immunotherapy in relation to reirradiation (neoadjuvant, concurrent, adjuvant)? Third, what is the role of standard systemic therapy and other targeted treatments in this paradigm? Fourth, will the addition of immunotherapy allow for a dose reduction in radiation, thereby reducing the risk of exceeding dose thresholds to the lung and mediastinal structures? And finally, how much, if any, will the combination treatment improve survival outcomes, and will this differ with SABR vs. conventionally fractionated radiation? Prospective, and ideally randomized, trials addressing these questions would be of great utility.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Study of Pembrolizumab (MK-3475) Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non-Small Cell Lung Cancer (MK-3475-024/KEYNOTE-024). Accessed November 15, 2016. Cited February 23, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02142738
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Gettinger SN, Zhang X, Homer R, et al. Pneumonitis in non-small cell lung cancer (NSCLC) patients treated with programmed death 1 (PD1) axis inhibitors. J Clin Oncol 2016;34:abstr 9030.
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281-93. [Crossref] [PubMed]
- Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Cohen JV, Kluger HM. Systemic Immunotherapy for the Treatment of Brain Metastases. Front Oncol 2016;6:49. [Crossref] [PubMed]
- Moscetti L, Nelli F, Felici A, et al. Up-front chemotherapy and radiation treatment in newly diagnosed nonsmall cell lung cancer with brain metastases: survey by Outcome Research Network for Evaluation of Treatment Results in Oncology. Cancer 2007;109:274-81. [Crossref] [PubMed]
- Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res 2007;13:2158-67. [Crossref] [PubMed]
- Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol Res 2016;4:124-35. [PubMed]
- Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013;86:343-9. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]
- Weber JS, Amin A, Minor D, et al. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res 2011;21:530-4. [Crossref] [PubMed]
- Queirolo P, Spagnolo F, Ascierto PA, et al. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 2014;118:109-16. [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Patel KR, Lawson DH, Kudchadkar RR, et al. Two heads better than one? Ipilimumab immunotherapy and radiation therapy for melanoma brain metastases. Neuro Oncol 2015;17:1312-21. [Crossref] [PubMed]
- Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012;117:227-33. [Crossref] [PubMed]
- Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 2013;23:191-5. [Crossref] [PubMed]
- Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013;2:899-906. [Crossref] [PubMed]
- Du Four S, Wilgenhof S, Duerinck J, et al. Radiation necrosis of the brain in melanoma patients successfully treated with ipilimumab, three case studies. Eur J Cancer 2012;48:3045-51. [Crossref] [PubMed]
- Du Four S, Hong A, Chan M, et al. Symptomatic Histologically Proven Necrosis of Brain following Stereotactic Radiation and Ipilimumab in Six Lesions in Four Melanoma Patients. Case Rep Oncol Med 2014;2014:417913. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res 1999;59:6028-32. [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003;170:6338-47. [Crossref] [PubMed]
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009;10:718-26. [Crossref] [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. [Crossref] [PubMed]
- Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002;62:1462-70. [PubMed]
- Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589-602. [Crossref] [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol 2007;25:4146-52. [Crossref] [PubMed]
- Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64:192-6. [Crossref] [PubMed]
- Milton DT, Miller VA. Advances in cytotoxic chemotherapy for the treatment of metastatic or recurrent non-small cell lung cancer. Semin Oncol 2005;32:299-314. [Crossref] [PubMed]
- Noble J, Ellis PM, Mackay JA, et al. Second-line or subsequent systemic therapy for recurrent or progressive non-small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2006;1:1042-58. [Crossref] [PubMed]
- Patel NR, Lanciano R, Sura K, et al. Stereotactic body radiotherapy for re-irradiation of lung cancer recurrence with lower biological effective doses. J Radiat Oncol 2015;4:65-70. [Crossref] [PubMed]
- Reyngold M, Wu AJ, McLane A, et al. Toxicity and outcomes of thoracic re-irradiation using stereotactic body radiation therapy (SBRT). Radiat Oncol 2013;8:99. [Crossref] [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [Crossref] [PubMed]
- Jackson MA, Ball DL. Palliative retreatment of locally-recurrent lung cancer after radical radiotherapy. Med J Aust 1987;147:391-4. [PubMed]
- McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014;90:819-27. [Crossref] [PubMed]
- Montebello JF, Aron BS, Manatunga AK, et al. The reirradiation of recurrent bronchogenic carcinoma with external beam irradiation. Am J Clin Oncol 1993;16:482-8. [Crossref] [PubMed]
- Okamoto Y, Murakami M, Yoden E, et al. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys 2002;52:390-6. [Crossref] [PubMed]
- Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260-6. [Crossref] [PubMed]
- Wu KL, Jiang GL, Qian H, et al. Three-dimensional conformal radiotherapy for locoregionally recurrent lung carcinoma after external beam irradiation: a prospective phase I-II clinical trial. Int J Radiat Oncol Biol Phys 2003;57:1345-50. [Crossref] [PubMed]
- Zimmermann FB, Molls M, Jeremic B. Treatment of recurrent disease in lung cancer. Semin Surg Oncol 2003;21:122-7. [Crossref] [PubMed]
- Sumita K, Harada H, Asakura H, et al. Re-irradiation for locoregionally recurrent tumors of the thorax: a single-institution, retrospective study. Radiat Oncol 2016;11:104. [Crossref] [PubMed]
- Griffioen GH, Dahele M, de Haan PF, et al. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer 2014;83:356-62. [Crossref] [PubMed]
- Chao HH, Berman AT, Simone CB 2nd, et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:281-92. [Crossref] [PubMed]
- Topkan E, Yildirim BA, Guler OC, et al. Safety and palliative efficacy of single-dose 8-Gy reirradiation for painful local failure in patients with stage IV non-small cell lung cancer previously treated with radical chemoradiation therapy. Int J Radiat Oncol Biol Phys 2015;91:774-80. [Crossref] [PubMed]
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210-25. [Crossref] [PubMed]


