Do we really care about incidental lung nodules?—Review of atypical lung carcinoid and a proposal for systematic patient follow up
Introduction
Atypical carcinoids (ACs) of the lung are intermediate-grade neuroendocrine tumors (NET) with malignant potential. Lung ACs are often greater than 0.5 cm in size and originate from naturally occurring neuroendocrine cells of the tracheobronchial epithelium (1). They are more prevalent in older females with a predominance in Caucasians (2,3). Rarely, they are seen in the pediatric population. Patients are often asymptomatic or may present with vague pulmonary symptoms like cough, hemoptysis, wheezing, or recurrent pneumonia (3). Patients may also present with symptoms of Cushing syndrome and carcinoid syndrome (bronchospasm, flushing, and diarrhea). Carcinoid syndrome symptoms signify carcinoid metastasis to the liver (4). The 50% of all patients with ACs have lymph node metastases at the time of due to its indolent course (5). The 5-year survival rate is inversely related to the tumor stage at presentation. The rate of metastases and recurrence is worse for ACs compared to typical lung carcinoids (TCs) (2,4,5). ACs may be detected as an incidental central or peripheral pulmonary nodule. Histopathology and immunohistochemistry (IHC) is often required to confirm the diagnosis. Metastases usually occur to the liver, bones, adrenals, and brain. Most of the patients have metastases at the time of presentation, with chemoradiation as the only possible treatment option. Surgical resection is optimal for a solitary lesion, like in the present case.
Since initial disease is asymptomatic, patients do not seek medical care until it metastasizes. This article is written with an intent to educate readers about the crucial role of close patient follow-up (Table 1). This article proposes sending patients reminder letters after discovery of a pulmonary nodule to increase patient awareness and, hopefully, compliance.
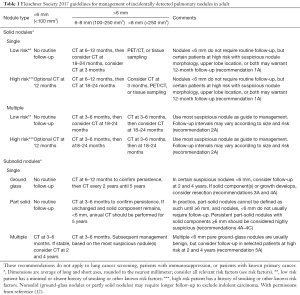
Full table
Case presentation
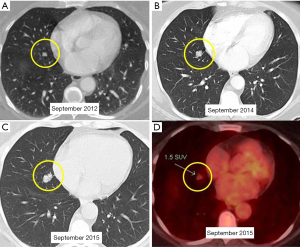
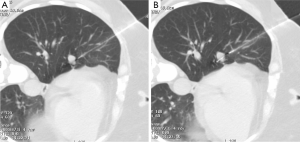
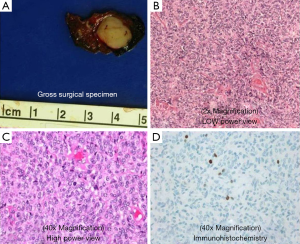
A 52-year-old lifetime non-smoker female with no significant past medical and surgical history presented for a routine follow-up of a right lower lobe solitary pulmonary nodule. This nodule was incidentally detected on a CTA chest performed in 2012 to rule out a pulmonary embolus (Figure 1). After the initial scan in 2012, the patient was lost to follow-up for two years and presented again in 2014. CT scan of the chest now showed that the right lower lobe non-calcified pulmonary nodule had grown in size (Figure 1B). A one year follow-up with a CT scan of the chest showed that the lung nodule had doubled in size over three years, highly suspicious for malignancy (Figure 1C). PET/CT showed mild uptake in the enlarging pulmonary nodule (Figure 1D). Subsequently, a CT-guided biopsy of the nodule revealed a carcinoid tumor (Figure 2). Next, the patient underwent a right middle lobectomy with an uncomplicated recovery (Figure 3A). Pathology results showed a well-differentiated NET with more than 2 mitoses per 10 hpf with no atypia or necrotic foci (Figure 3B,C), consistent with an atypical lung carcinoid. Tumor cells stained positive for cytokeratin 7, TTF-1, chromogranin A (CgA), synaptophysin, and negative for CK20, with Ki-67 proliferation index of 1% (Figure 3D), consistent with a lung carcinoid. Follow-up imaging showed no local spread or distant metastases. The patient is disease free till date, consistent with cure.
Discussion
NETs occur along a spectrum of malignancies, the most malignant of which is small cell lung cancer (2). NETs are most commonly described in the gastrointestinal tract and lung (3). There is an increased incidence in women and whites compared to men and other ethnicities (2). They most commonly occur in the 40–60 years age group with the mean age at 45 years for ACs, ten years younger than the average age for TCs (2). Annual incidence of NETs has increased from 0.3 cases in 1973 to 1.35 cases per 100,000 in 2004, most likely the result of improved detection techniques (2,3). Lung NETs are divided into four varieties: low-grade/well-differentiated (typical), intermediate-grade (atypical), high-grade large cell (poorly-differentiated), and high-grade small cell (poorly differentiated) (3). Collectively, lung NETs make up 25% of primary lung carcinomas (3). Intermediate-grade ACs, the topic of this discussion, are the rarest subtype with an incidence <1% (3). ACs have not shown an association with smoking and about 5% are associated with MEN1 (3).
Diagnosis and classification of NETs is challenging. The role of IHC and imaging are used to aid in the diagnosis. All NETs show the typical neuroendocrine morphology in the form of Kulchitsky cells in small clusters or rarely, isolated and ACs are required to show an additional 2–10 mitoses per 2 mm2 of viable area of tumor with necrosis (4). Necrosis is typically located at the center of the tumor nodule (4). Mitoses <2 would signify a TC and >10 would signify LCNEC (4). Carcinoid tumors can be diagnosed with cytological assessment, but differentiating between ACs and TCs requires a resected specimen (4). IHC can strengthen the diagnosis in cases of equivocal histology. Common NET IHC markers are chromogranin A (CgA), CD56, synaptophysin, and neuron-specific enolase. CgA can be used to detect tumor recurrence as it is associated with tumor burden, but this should be done with caution as unrelated factors can cause CgA levels to rise (4). Since the release of the 7th edition of TNM staging criteria designed by the American Joint Committee on Cancer, carcinoid tumors now have TNM parameters (same as for NSCLC) (4). Interpretation of the minor histological differences between the various NETs leads to interobserver variability and delays accurate diagnosis (3). ACs are more likely to have delayed diagnosis because more than 90% usually present as non-functional (no hormone production) incidental peripheral lung tumors (5). In a study about patient perspective of their NET diagnosis, 50% of the 222 patients reported a period up to two years between symptom presentation and diagnosis (6). In a 2015 study, ACs were shown to be more aggressive than TCs with poorer 5- and 10-year survival rates (7). The delay in diagnosis allows for local tumor invasion as well as distant osseous and hepatic metastases. Imaging is used to localize and stage the tumor. According to the European Society of Medical Oncology, CT or MRI should be performed annually in patients with AC/TC who have had surgical resection or every three months after initiating medical treatment (8). What imaging cannot do is different between ACs and TCs—this requires nuclear imaging. Somatostatin receptor scintigraphy (SR) with radiolabeled somatostatin analogue (SSA) would not only help find tumor sites, but it would prove the presence of SRs and help narrow down medical management options (9). PET scans with 68Ga-DOTATATE and 68Ga-DOTATOC have a higher sensitivity and specificity than the octreoscan for SRs imaging (9). 18F-fluorodeoxyglucose PET/CT has been a suggested imaging modality for tumors with a Ki-67 index >10% (8). Radiologists have the ability to play a vital role in the care of patients from diagnosis to treatment to post-treatment monitoring. The current grading protocol classifies NETs as grade 1 (TCs), grade 2 (ACs), and grade 3 (SCLCs and LCNECs). Hormonally active grade 1 and 2 tumors are usually started on SSA treatment (2,5). SSA resistant tumors may benefit from additional serotonin synthesis inhibitor, telotristat etiprate (10). Grade 3 tumors are initially treated with chemotherapy (3). There is also recommendation for use of adjuvant chemotherapy in stage II/III AC patients. Ultimately, surgical resection is the only curative option for patients with resectable tumors or minimally metastatic disease. The goal should be tumor removal with maximal lung parenchyma preservation. AC patients status post resection continue to have a lower 5- and 10-year survival rate compared to TC patients due to increased risk of metastases and recurrence (2,4,5). The increased rate of nodal involvement with ACs warrants lymph node dissection even in patients with N0 disease (11). Current guidelines created by the Fleischner Society (Table 1), direct radiologists for follow up of incidental pulmonary nodules, which is often how NETs are discovered (12). However, the degree of adherence to these guidelines remains largely unexplored. A quality improvement study at a major academic tertiary hospital discovered that their radiologists’ adherence to the Fleischner guidelines was 82.8% which is relatively higher than rates reported at other institutions. Researchers attribute the increased adherence rates to a laminated printout of the Fleischner guidelines at each PACS station and reinforcement through resident learning events (13). These types of departmental efforts to reinforce the Fleischner guidelines are not readily implemented at every institute. One study found that only 29% of emergency department (ED) CT pulmonary angiography reports that explicitly stated a follow-up protocol of an incidental pulmonary nodule were actually followed up (14). There are greater systemic issues at play than the radiology report when it comes to poor patient follow-up: inadequate interdepartmental communication, pressure for rapid patient turnover, radiologists’ inattention to detail, irrelevance of finding to acute condition, lack of primary care access, and patient demographics (14). These are real-life problems that lead to poor follow-up and can have grave consequences for patients (14). The purpose of this case report is to reinforce the importance of follow-up in a patient who is diagnosed with a lung nodule and offer an improved form of follow-up communication inspired by the post-mammography patient correspondence protocol at our institute. We propose sending a letter to the patient in layman terms, directly from the Radiology Department to reinforce the importance of timely follow up, which will complement the information provided to the patient from their primary care physician or pulmonologist’s office.
Conclusions
Patient loss to follow-up after an incidental finding of a pulmonary nodule can have devastating consequences, as not all patients with NETs can be as lucky as the patient in the present case. We propose sending patients with pulmonary nodules a standardized reminder lay letter from the Radiology Department to inform them of the findings and emphasize the importance of follow-up. Radiology administrative staff can be trained to send an appropriate letter based on the patient’s nodule size. Additionally, radiology staff can communicate with the patient’s primary care physicians or pulmonologist complementing their own follow-up protocols. The end result will be that the patient will be better informed of their diagnosis and management plan.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hilal T. Current understanding and approach to well differentiated lung neuroendocrine tumors: an update on classification and management. Ther Adv Med Oncol 2017;9:189-99. [Crossref] [PubMed]
- Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol 2017;12:425-36. [Crossref] [PubMed]
- Naalsund A, Rostad H, Strøm EH, et al. Carcinoid lung tumors--incidence, treatment and outcomes: a population-based study. Eur J Cardiothorac Surg 2011;39:565-9. [Crossref] [PubMed]
- Wolin EM. Challenges in the Diagnosis and Management of Well-Differentiated Neuroendocrine Tumors of the Lung (Typical and Atypical Carcinoid): Current Status and Future Considerations. Oncologist 2015;20:1123-31. [Crossref] [PubMed]
- Detterbeck FC. Clinical presentation and evaluation of neuroendocrine tumors of the lung. Thorac Surg Clin 2014;24:267-76. [Crossref] [PubMed]
- Granberg D, Sissons M, Kolarova T, et al. Lung neuroendocrine tumor (NET) patient (pt)-reported experience: Results from the first global NET pt survey: A collaboration between the international neuroendocrine cancer alliance (INCA) and Novartis pharmaceuticals. J Clin Oncol 2015;33:abstr e17739.
- Filosso PL, Ferolla P, Guerrera F, et al. Multidisciplinary management of advanced lung neuroendocrine tumors. J Thorac Dis 2015;7:S163-71. [PubMed]
- Hörsch D, Schmid KW, Anlauf M, et al. Neuroendocrine tumors of the bronchopulmonary system (typical and atypical carcinoid tumors): current strategies in diagnosis and treatment. Conclusions of an expert meeting February 2011 in Weimar, Germany. Oncol Res Treat 2014;37:266-76. [Crossref] [PubMed]
- Phan AT, Oberg K, Choi J, et al. NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas 2010;39:784-98. [Crossref] [PubMed]
- Kulke MH, O'Dorisio T, Phan A, et al. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer 2014;21:705-14. [Crossref] [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Eisenberg RL. Fleischner Society. Ways to improve radiologists' adherence to Fleischner Society guidelines for management of pulmonary nodules. J Am Coll Radiol 2013;10:439-41. [Crossref] [PubMed]
- Blagev DP, Lloyd JF, Conner K, et al. Follow-up of Incidental Pulmonary Nodules and the Radiology Report. J Am Coll Radiol 2016;13:R18-24. [Crossref] [PubMed]


