Circulating tumor cell interactions with macrophages: implications for biology and treatment
Introduction
In most cases, mortality in cancer patients is linked to metastatic disease (1). Under such circumstances, surgical intervention is no more curative and, unfortunately, the metastases may have proceeded to a chemoresistant state which cannot be controlled in most tumor entities (2). Tumor dissemination is a complex process which is often described as a series of discrete steps comprising activation of migrating subpopulations of tumor cells, intravasation, survival in the circulation and extravasation at distal sites to establish secondary lesions. Clearly, tumor cells prime the surrounding microenvironment to support progression and cell spread which involves participation of different normal cell types in the tumor stroma. Especially, tumor-associated macrophages (TAMs) seem to play a key role in activating dissemination and providing protection against the immune system and, possibly, likewise against chemotherapy (3). Characteristics of TAMs are intensely investigated in respect to functional polarization, cytokines produced and possible therapeutic targets to switch their protumor role to the usual antitumor effector function. Tumor dissemination is initiated by movement of specialized cancer cells into the circulation or the lymphatic system which are generally described as circulating tumor cells (CTCs) (4). CTCs have been recognized for a long time but studies of these cells have been hampered by relative scarcity in most tumor types and heterogenous phenotypes. In particular, the great majority of CTCs perish in the circulation and the characteristics of the small subpopulation of actual metastasis-initiating cells (MICs) among the CTCs are not known so far (5).
Lung cancer is still the leading cause of cancer-related death in the world, and there are about 1.5 million newly diagnosed lung cancer cases each year (6). Small cell lung cancer (SCLC) accounts for approximately 15% of lung cancers and represents an aggressive tumor with high numbers of CTCs which are involved in early dissemination (7). The unique properties of CTCs of ED-SCLC allowed us to expand six cell lines from blood samples of patients with metastatic disease in vitro (BHGc7, 10, 16, 26, 27 and UHGc5) and to study their cell biologic characteristics and interaction with normal cell populations of the tumor microenvironment (8). SCLC disseminates rapidly such representing a suitable tumor model of both cancer spread and development of general chemoresistance. This review summarizes the new findings obtained employing the novel CTC SCLC cell lines and their implications for the treatment of SCLC which has not been improved for the last decades (9).
TAMs
Rudolf Virchow found a characteristic link between tumors and infiltrating leukocytes in 1863 and, subsequently, it became clear that tumor initiation and progression is influenced by interactions between tumor and invading normal cells (10-12). Several stromal cell types become activated and form a pro-tumorigenic microenvironment. Hematopoietic cells are recruited to most tumors and one group, the TAMs, can constitute a large portion of the tumor mass (13,14). Macrophages produce an array of cytokines, chemokines, polypeptide growth factors, hormones, matrix remodeling proteases and metabolites, many of which possess tumor-promoting and tumor-protecting activities (15-17). For many solid tumor types, high densities of cells expressing macrophage-associated markers have generally been found to associate with poor clinical outcome (18-20).
In particular, TAMs exhibit tumor-supporting effects in response to stimulation or education by cytokines in many solid tumors instead of fighting cancer cells (13,21,22). TAMs are either tissue resident or derived from peripheral sources such as monocytes of bone marrow and spleen (23). The tumor margin is an active site of immune cell-tumor interactions where TAMs at the leading edge seem to drive invasive cellular phenotypes (13). Accumulation of TAMs is observed in regions of hypoxia in growing tumors and their recruitment is mediated by an upregulation of macrophage chemoattractants, including endothelin-2 (ET-2) and vascular endothelial growth factor (VEGF) (20). Local conditions in these regions correlate with a switch in macrophage polarization, neoangiogenesis and the subsequent acquisition of an invasive phenotype (24,25). Inhibition of the matrix metalloproteinase-9 (MMP9) in macrophages blocked the release of VEGF and thereby inhibited angiogenesis and tumor growth (26).
Furthermore, in breast cancer and glioma, TAMs facilitated tumor cell invasion through a paracrine signaling loop that involves tumor-derived colony-stimulating factor 1 (CSF-1) and macrophage-derived epidermal growth factor (EGF) as wells proteases, such as cysteine cathepsins (27,28). Tumors and, possibly, CTCs educate macrophages to promote invasion, intravasation as well as survival in the circulation and durable growth at secondary lesions (3,29,30). The invasiveness of tumor cells was dramatically enhanced when they were cocultured with macrophages or macrophage conditioned medium (31). TAMs can also be reprogrammed by various pharmacological agents and TAM-specific inactivation of IKK-β, which disrupts NF-κB signaling, resulting in a protumor polarization recovery, recruitment of natural killer (NK) cells and subsequent tumor regression in an ovarian cancer model (21). These findings show that tumor-macrophage interactions are instrumental in tumor dissemination although the mechanisms by which TAMs acquire pro-metastatic abilities have not been fully characterized.
Inflammation and SCLC
SCLC is an aggressive neuroendocrine malignancy characterized by aggressive growth and early development of metastases (7,32). This tumor responds initially excellent to platinum-based chemotherapy in most cases but invariably relapses rapidly with a dismal prognosis (7,33). Contrary to increasing 5-year survival rates for other solid cancers, therapeutic options for SCLC have remained unchanged for the last decades, with minor improvement in outcomes (34). The camptothecin topotecan, the single drug approved for second-line treatment of SCLC, exhibits low response rates of short duration (35). Despite putative actionable genetic alterations in half of the SCLC patients targeted clinical trials has yielded no progress so far (13,32,36). SCLCs show inactivation of p53 and retinoblastoma (RB1) suppressor genes and, therefore, a range of distinct growth factors result in intense progression (13).
Drug resistance is the most important cause of failure of SCLC chemotherapy (33,37). Early thoracic irradiation with concurrent etoposide/cisplatin chemotherapy is state-of-the-art treatment for limited disease small cell lung cancer (LD-SCLC) achieving 5-year survival rates of at least 20% (38,39). In terms of ED-SCLC, a median survival time of 9–13 months and 2-year survival of 5–20% are still unsatisfactory (33,40,41). Despite extensive basic and clinical research over the past 30 years, little progress has been made in treating SCLC (33,35,42).
Patients with COPD are at increased risk for developing SCLC, indicating a promotion of mutated malignant cells by the inflammatory conditions (43,44). COPD is characterized by an accumulation of airway macrophages (AMs) which are activated by cigarette smoke and other irritants to release inflammatory mediators, such as tumor necrosis factor (TNF-α), IL-8, other CXC chemokines, monocyte chemoattractant protein-1 (MCP-1), LTB4, and reactive oxygen species (45). These factors in turn release chemotactic mediators which recruit additional inflammatory cells causing chronic inflammation, which results in airway obstruction and respiratory symptoms. Destruction of lung parenchyma is effected by proteases secreted by AMs, such as MMPs and several cathepsins (46). The lung macrophages found in abundance in COPD display a mixed phenotype of both M1 and M2 markers (47). Most of the inflammatory proteins in COPD macrophages are regulated by NF-κB which is activated in AMs of COPD patients (48).
Epidemiological and clinical studies have suggested that chronic inflammation promotes and aggravates malignancy (49,50). About 25% of all cancers are etiologically linked to chronic inflammation and infection (51). Presence of COPD increases the incidence of lung cancer and lung cancer death (52). COPD is established as the single most important risk factor for lung cancer after smoking exposure (53). The inflammation present in the tumor microenvironment is characterized by variable leukocyte infiltration, comprising TAMs, mast cells, dendritic cells, NK cells, neutrophils, eosinophils and lymphocytes. These cells produce a variety of cytotoxic mediators such as reactive oxygen and nitrogen species, proteases, membrane perforating agents, MMPs, TNFα, interleukins (IL-1, IL-6, IL-8), interferons (IFNs) and enzymes, as cyclooxygenase-2 (COX-2), lipooxygenase-5 (LOX-5) and phospholipase A2 (PLA2), which activate or are activated by transcription factors as NF-κB and STAT3 (54). Accordingly, the use of anti-inflammatory agents is associated with a reduced incidence of colorectal, breast, pancreatic, and gastric cancers (55,56). Thus, chronic inflammation preceding and associated with SCLC may constitute an important factor in generation of a large number of CTCs and, possibly, chemoresistance.
SCLC and CTCs
CTCs are rare events in most tumor types, with a frequency of approximately one CTC among 1–10 million mononuclear blood cells (4,57). Therefore, these cancer cells have to be enriched by various methods for further analysis. However, SCLC patients can exhibit extreme numbers of CTCs which have prognostic significance and correlate with responses to chemotherapy (58-60). For example, CTCs were identified in 90% (54/60) of patients at baseline, with CTC reaching numbers of up to 24,281/7.5 mL blood as determined using the CellSearch® system (61). CTC count was strongly correlated with the number of organs bearing metastases. A reduction of CTC count exceeding 89% in response to chemotherapy was associated with a lower risk of death (HR =0.24; 95% CI, 0.09–0.61). Similarly, lower numbers of CTCs were observed for 21 patients with LD-SCLC (median =6; range, 0–220) compared with 38 patients with ED-SCLC (median =63; range, 0–14,040) and the absence of CTCs in 27% of patients was correlated with prolonged survival (HR =3.4; P≤0.001) (62).
The release of CTCs has been designated “shedding”, indicating a lack of detailed knowledge about this process. Even small tumors are reported to shed millions of cancer cells (63). An estimation of the release of CTCs from solid tumors at a high daily rate of 3.2×106 to 4.1×106 per gram of tissue is based on a rat tumor model (64). The shedding of MTW9 rat mammary carcinoma cells comprised about 10% of the tumor weight and resulted in a CTC count of approximately 20,000 CTCs/mL blood. Clearly this determination of the release of CTCs reported from an experimental animal model is not valid for human tumors. A threshold of 3–5 CTCs/7.5 mL blood has been defined by the CellSearch® system for breast, prostate and colon cancer patients with favorable or poor prognosis, respectively, which seems not compatible with millions of intravasated cancer cells (65). In cancer patients, half of all CTCs perish within 2.4 h, although longer half-lives were reported in other studies (66,67).
The actual mechanisms of tumor cell shedding are not known so far. CTCs seem to origin as specialized cell type, different from the bulk of the tumor cells, from the margin of the tumors. CTCs leave the particular microenvironmental milieu characterized by inflammation, acidosis and hypoxia through the interaction of a host of participating cell types. Therefore, CTCs are not expected to represent the bulk of tumor cells and are not typical of the cell biologic behavior of the main body of the tumor. Most of the CTCs populations analyzed exhibit heterogeneity, pointing to release of different cell populations from distinct regions of the tumor or from metastatic lesions. Out of all CTCs shed by the primary tumor only about 0.1% survives in the circulation and less than 0.01% is capable of forming secondary lesions (57,68,69). This attrition has been attributed to shear stress and an unfavorable microenvironmental conditions in the blood. Furthermore, the vast majority of CTCs are likely to become trapped in various capillary beds. Consistent with a degradation of CTCs in the periphery, a lot of CTC-associated materials are detectable in tumor patients.
Multiphoton intravital imaging techniques have been used to observe macrophage-tumor interactions during metastatic dissemination in live animals (70). Macrophages are localized primarily near the tumor margin and decrease in number toward the center. Local or recruited TAMs localize to blood vessels, where they seem to help tumor cells intravasate into the circulation. Since macrophages seem to promote release of CTCs and invasion, preexisting inflammation as in COPD may be one of the factors responsible for the extremely high numbers of CTCs in SCLC patients. A second type of cancer associated with high CTC counts is inflammatory breast cancer (IBC). CTC count among 147 patients with IBC reached 179 and 249 CTCs/7.5 mL blood for patients with stage III and metastatic IBC, respectively (71). IBC patients with CTCs in the peripheral blood had abnormalities in adaptive immunity that may impact tumor cell dissemination (72). IBC carcinoma tissues exhibit marked monocytes/macrophages infiltrates localized around tumor emboli (73). This influx of macrophages within the IBC tumor microenvironment correlated with increase in the number of positive lymph node metastasis and expression of cathepsins and MMPs. The processes associated with tumor spread could not be studied in detail as cells determined to disseminate the tumor, namely CTCs, are scarce in blood and could not be kept and expanded in tissue culture except for one case of a colon CTC line and several breast cancer CTC lines, established recently (74,75). We have set up a total of six SCLC CTC lines so far and the first two permanent CTC lines, namely BHGc7 and 10, were used to study CTC/macrophage interaction in cocultures (8).
CTC-Macrophage Interaction
For tumor-macrophage interactions the responsible cytokines and factors are well characterized but the specific contributions of CTCs has been not known so far. The first establishment of two permanent CTC lines from blood samples of advanced stage SCLC patients allowed us to investigate the CTC-immune cell interaction (8). Cocultures of peripheral blood mononuclear cells (PBMNCs) with CTC lines BHGc7 and 10 or addition of CTC-conditioned medium in vitro resulted in monocyte-macrophage differentiation and appearance of CD14+, CD163weak and CD68+ macrophages expressing markers of TAMs (76,77). Macrophages recruited by the metastatic SCLC26A cell line established from a pleural effusion showed expression of osteopontin (OPN), MCP-1, IL-8, chitinase-3-like-1 (CHI3L1), platelet factor (Pf4), IL-1ra, and MMP-9, among other minor cytokines/chemokines. In contrast, BHGc7-CM induced marked overexpression of complement factor D (CFD)/adipsin and vitamin D-BP (VDBP), as well as increased secretion of OPN, lipocalin-2 (LCN2), CHI3L1, uPAR, MIP-1 and growth differentiation factor 15 (GDF-15)/MIC-1. BHGc10, derived independently from relapsed SCLC, revealed an almost identical pattern with added expression of ENA-78/CXCL5. OPN expressed by macrophages has been implicated in cytokine expression, phagocytosis, migration and tumor recurrence and MCP-1 expression is a significant indicator of early relapse (78-83). CHI3L3 was described as typical marker of M2 macrophages in mice (84). According to our results, CHI3L1 pseudo-chitinase is the corresponding counterpart in humans where it constitutes an important regulator of inflammation, angiogenesis and M2 macrophage differentiation in addition to its expression by SCLC CTCs (85,86). Pf4/CXCL4 has been demonstrated to prevent monocyte apoptosis and to promote macrophage differentiation from peripheral blood monocytes (87-89). CFD is essential for alternative pathway activation and was found to be identical to the adipokine adipsin which is expressed in monocytes/macrophages (90-92). VDBP is as precursor of the group-specific component protein-derived macrophage-activating factor (GcMAF) (93). LCN2 plays an important role in promoting cell migration and invasion in cooperation with MMP-9 (94). GDF-15/MIC-1 is associated with aberrant growth and a poor prognosis (95). Macrophage inflammatory proteins (MIPs) lead to acute neutrophilic inflammation (96). In conclusion, tumor and CTC-leucocyte interactions induce monocyte-macrophage differentiation and elicit a host of mediators which promote further recruitment of inflammatory cells, stroma breakdown and invasion.
CHI3L1 in inflammation and cancer
CHI3L1/YKL-40 is a secreted 40 kDa glycoprotein that is up-regulated in several human cancers and other diseases characterized by chronic inflammation (97). CHI3L1 is expressed by a range of normal cells, including macrophages, neutrophils, epithelial cells, smooth muscle cells, and chondrocytes, and its expression is enhanced by a number of cytokines, including IL13, IL6, IL1β, and IFNγ (98,99). Increased serum levels of CHI3L1 have been associated with a negative prognosis in nonmalignant diseases such as inflammation and asthma (99,100). Bronchial epithelial cells treated with CHI3L1 showed a significant increase of IL-8 production, which was dependent on MAPK (JNK and ERK) and NF-κB pathways activation (101). YKL-40-induced IL-8 was found to further stimulate proliferation and migration of BSMCs.
Elevated levels of YKL-40 in the circulation were found in a number of solid tumors including glioblastoma, breast, colon, lung, prostate, bladder, stomach and endometrial cancer, esophageal squamous cell carcinoma, liver, pancreas, head and neck cancer, and others (102). Furthermore, CHI3L1 has an important role in cancer progression, and high CHI3L1 expression is associated with disease severity and shorter overall survival in many types of cancers. High CHI3L1 expression was independently associated with poorer overall survival in both NSCLC and SCLC patients (103). Serum CHI3L1 levels in 120 SCLC patients were higher in the group with a poorer response to chemotherapy and those revealing a shorter survival (104).
Macrophages and the SCLC26A cell line express CHI3L1, an important regulator which lacks chitinase enzyme activity (8,86,102). Screening of the cytokines/chemokines in the six SCLC CTC lines which were established from distinct ED-SCLC patients (BHGc7, 10, 16, 26, 27 and UHGc5) revealed abundant expression CHI3L1, VEGF and MMP9 in Western blots and ELISA tests (manuscript submitted). CHI3L1-positive tumor cells, exhibiting increased expression of VEGF and MMP9, display the combination of characteristics required to generate metastases as CTCs. A member of the family of chitinases, chitinase 1 (CHIT1), is both an immune mediator and a regulator of tissue remodeling in lung diseases such as COPD (105). Furthermore, CHIT1 promoted the release of IL-8, MCP-1, and MMP-9. The most prominent cytokine/chemokine detected in the SCLC CTC supernatants CHI3L1 is also known as YKL-40 (86). CH3L1 pseudo-chitinase, which exhibits an inactivated enzymatic site but has retained chitin-binding activity, functions as regulatory factor of normal cell types, including macrophages (106). CHI3L1/YKL-40 has been described as soluble marker of invasive cancers with poor prognosis for a wide range of malignancies including lung cancer and, particularly, SCLC. No CHI3L1/YKL-40 mRNA expression was described for the SCLC cancer cells, whereas YKL-40 expression was present in all tumors in macrophages localized in the peritumoral stroma (107). CHI3L1 is a downstream product of STAT3 and selected tumor cells seem to be induced by inflammatory cytokines secreted by TAMs, most likely in close contact with these immune effector cells in the peritumoral stromal tissue (86).
CHI3L1 production of expanded SCLC CTCs point to these disseminated tumor cells as source of this protein directly released into blood (86). Whereas in tumors with counts of several CTCs/mL their contribution of CHI3L1 may be negligible, in tumors which can exhibit counts exceeding several hundred tumor CTCs/mL blood, such as SCLC, CTCs may be the major source of this cytokine (60,108). However, determinations of serum CHI3L1 in cancer patients may be not tumor-specific due to secretion from inflamed normal tissues. High expression of CHI3L1 in solid tumors, as indicator of dissemination and poor prognosis, may at least partially be due to CHI3L1-positive CTCs (109).
The interactions of CHI3L1 with pathways involved in tumor cell progression, survival and metastasis are subject of intense research (Figure 1) (102). Preclinical studies and experimental models using glioma cells indicate a role of CHI3L1 in tumor dissemination and drug resistance (110). The ability of CHI3L1 to bind heparan sulfates allows its interaction with cell surface proteoglycan syndecan-1, resulting in VEGF production and enhanced angiogenesis in glioblastoma (110). Lung metastasis of melanoma and breast cancer cells was reduced after blocking ChI3L1 with antibodies or removing its regulator semaphorin 7a in experimental animals (98). Treatment with chitin microparticles inhibited tumor growth and angiogenesis at primary tumor sites but also reduced metastasis to the lung (106,111). CHI3L1 was also shown to bind the surface “Receptor for Advance Glycation End” Product (RAGE) which supports progression of solid tumors through promotion cell proliferation, migration and survival (112). Interaction of CHI3L1 with RAGE stimulates growth of intestinal epithelial cell (IEC) through STAT3-, β-catenin- and NF-κB-associated signaling pathways. Similarly, the binding of CHI3L1 to IL13Rα2 results in the activation of ERK1/2, AKT, and Wnt/β-catenin signaling (113). Semaphorin 7a appears to activate CHI3L1 via integrin-β1 or decreases its activity via plexin C1 (102). Melanoma lung metastasis was impaired in the absence of IL13Rα2 (102). Thus, CHI3L1 seems to represent an important mediator and regulator of local inflammation in benign and malignant diseases and important promoter of angiogenesis, invasion and metastasis expressed in primary tumors, immune cells and CTCs. The molecular interactions of CHI3L1 are involved in metastasis and offer new therapeutic targets for inhibiting tumor dissemination (114).
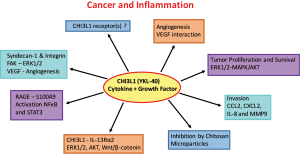
Mechanisms of chemoresistance in SCLC
SCLC responds well to the first cycles of platinum-based chemotherapy but relapses appear within approximately one year and exhibit universal chemoradioresistance (33). CTCs are supposed to show cancer stem cell (CSC)-like characteristics and to belong to the small subpopulation of cells which survive the initial cycles of chemotherapy due to increased chemoresistance (115). In contrast to this expectation, the first two SCLC CTC cell lines proved to be chemosensitive to cisplatin, etoposide and the second-line chemotherapeutics topotecan and epirubicin, with exception of cisplatin and BHGc10 established from a patient refractory to primary treatment (116). These findings have been confirmed in the four additional SCLC CTC lines (BHGc16, 26, 27 and UHGc5) newly established. Therefore, the use of CTCs as surrogate markers for the SCLC bulk tumor is questionable.
All six SCLC CTC cell lines established so far exhibit spontaneous formation of tumorospheres with diameters of up to 1–2 mm which provide protection of the cells by limited access of chemotherapeutics and quiescent and hypoxic tumor cells (117,118). Tumorospheres exhibit increased chemoresistance in vitro to cytotoxic drugs compared to the corresponding cells in form of single cell suspensions. This physical mechanism of SCLC chemoresistance explains the inefficiency of the broad range of drugs tried due to exclusion of any potential chemotherapeutic drug to reach the corresponding cellular target in susceptible cells in sufficient quantities. Whether multicellular cell aggregates exist in the primary tumors is not known. However, some SCLC tumor cells have survived the initial cycles of chemotherapy by mechanisms not known so far, possibly under the influence of the inflammatory environment. For example, TAMs are known to limit the cytotoxic effects of chemotherapy in preclinical models of cancer (119).
Inflammation mediators NF-κB and STAT3
Induction and continued secretion of CHI3L1 in normal cells require sustained activation of NF-κB (120,121). A range of human cancers have constitutive NF-κB activation due to the inflammatory microenvironment and oncogenic mutations (122). Furthermore, the interaction between NF-κB and STAT3 controls the communication between cancer cells and inflammatory cells and influences tumor angiogenesis and invasiveness (123-125). The persistent activation of STAT3 also mediates tumor-promoting inflammation (126,127). Coculture with malignant glioma cells stimulated monocytes to significantly upregulate expression of IL-10 and TGF-β, as well as of STAT3 (127). NF-κB in cancer maintains the immunosuppressive phenotype of TAMs and specific inhibition of NF-κB signaling in TAMs induces a switch to an antitumor phenotype and IL-12-dependent NK cell recruitment (128).
In vivo studies demonstrated that macrophages also mediate chemoresistance by supplying survival factors and/or activating anti-apoptotic programs in cancer cells. Co-cultures of mammary carcinoma cell lines and macrophages revealed macrophage-mediated STAT-3-dependent resistance to paclitaxel, doxorubicin and etoposide (28). Macrophage protection of tumor cells is in part dependent on cathepsin protease activity, specifically cathepsin B and S. Additionally, TNF-α may mediate chemoprotection, either directly through NF-κB activation, or indirectly IL-6 expression and STAT3 activation (129). Chemotherapeutic intervention, such as the use of cisplatin in SCLC may cause activation of multiple signal transduction pathways. Phosphorylation of NF-κB, mediated by the PI3/AKT signaling, was significantly enhanced upon cisplatin treatment (130). These studies point to therapeutic targets of the signaling pathways which regulate the downstream effector CHI3L1.
Conclusions
During tissue-repair in normal organs, macrophages promote healing by induction of neoangiogensis, generation of trophic signals, tissue remodeling and immunosuppression (3). In tumors, high TAM content is generally correlated with poor prognosis and CHI3L1 was reported to promote macrophage recruitment, polarization to protumor activity and angiogenesis (108,131,132). A microenvironment primed by COPD is rich in growth factors and cytokines that can stimulate proliferation and survival of the mutated premalignant cells, enabling them to accumulate further genetic changes as observed in SCLC. We studied the CTC-induced macrophages in respect to secreted cytokines/chemokines which are expected to be involved in promoting invasion of CTCs in SCLC (8). In general, invasiveness of tumor cells was dramatically enhanced when they were co-cultured with macrophages or macrophage-conditioned medium (31). These data suggest that SCLC CTCs seem to recruit and “educate” a specific type of macrophages operative in invasion, immune protection, extravasation and possibly cachexia (Figure 2). Thus, the well-established role of CTCs has to be extended to specific effects on monocyte-macrophage differentiation and specific priming (133,134). Intravasation is a significant rate limiting step in the metastatic process (69). Macrophages that interact with tumor cells may help to elicit a particular “invasion signature” of gene expression in these tumor cells (13). This co-option of macrophage functions in CTCs seems to include the important regulator CHI3L1 which is operational in invasion and tumor progression, besides VEGF and MMP9. Inhibition of CHI3L1 or its interacting proteins as well as binding to chitosan impairs metastasis in experimental animals and represent a new therapeutic target (102). Since the SCLC CTC lines proved to be chemosensitive, drug resistance seems to have been transiently induced in a subpopulation of the primary tumor cells, possibly under the influence of the inflammatory environment.
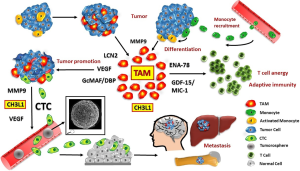
The detailed investigation of TAMs would require samples of tumor tissue but hardly any cases of SCLC are resectable. Putative contributions of CTCs at the site of their formation are not known for any kind of tumors. Availability of a panel of six SCLC CTC lines can be employed to extend the study of CTC-stroma cell interactions and the possible effects of inflammatory cytokines on cell biology and chemosensitivity of these cells. These CTC lines from ED-SCLC patients are highly similar in gene and protein expression as well as in respect to the release of a panel of cytokines. Therefore, these CTCs seem to present the actual MICs as selected in vivo from the initial heterogenous mixture of shedded tumor cells during relapses. In a putative two-step model the SCLC cells attract macrophages and polarize them for protumor assistance followed in turn by a macrophage-linked education of a fraction of tumor cells to generate invasive CTCs. Since we have previously found similar features of SCLC CTCs associated with other tumors, such as highly malignant glioblastoma, the findings reported here may potentially hold true for other malignancies as well (86).
Acknowledgements
We apologize to the many authors whose work we could not cite because of space constraints. We thank Dr. Doris Moser of the Department of Cranio, Maxillofacial, and Oral Surgery, Medical University of Vienna, for the SEM data. Furthermore, we wish to thank Dr. Theo B. Hohenheim for endorsement.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The respective ethics protocol was approved by the Medical University of Vienna Ethics Approval 366/2003.
References
- Mehlen P, Puisieux A. Metastasis:a question of life or death. Nat Rev Cancer 2006;6:449-58. [Crossref] [PubMed]
- Hamilton G, Rath B, Ulsperger E. A review of the role of surgery for small cell lung cancer and the potential prognostic value of enumeration of circulating tumor cells. Eur J Surg Oncol 2016;42:1296-302. [Crossref] [PubMed]
- Ostuni R, Kratochvill F, Murray PJ, et al. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 2015;36:229-39. [Crossref] [PubMed]
- Gkountela S, Szczerba B, Donato C, et al. Recent advances in the biology of human circulating tumour cells and metastasis. ESMO Open 2016;1:e000078. [Crossref] [PubMed]
- Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol 2012;198:281-93. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev 2015;29:1447-62. [Crossref] [PubMed]
- Hamilton G, Rath B, Klameth L, et al. Small cell lung cancer: Recruitment of macrophages by circulating tumor cells. Oncoimmunology 2015;5:e1093277. [Crossref] [PubMed]
- Koinis F, Kotsakis A, Georgoulias V. Small cell lung cancer (SCLC): no treatment advances in recent years. Transl Lung Cancer Res 2016;5:39-50. [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. [Crossref] [PubMed]
- Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol 2015;25:198-213. [Crossref] [PubMed]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263-6. [Crossref] [PubMed]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71-8. [Crossref] [PubMed]
- Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015;27:462-72. [Crossref] [PubMed]
- Hughes R, Qian BZ, Rowan C, et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res 2015;75:3479-91. [Crossref] [PubMed]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49-61. [Crossref] [PubMed]
- Takeya M, Komohara Y. Role of tumor-associated macrophages in human malignancies: friend or foe? Pathol Int 2016;66:491-505. [Crossref] [PubMed]
- Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia 2002;7:177-89. [Crossref] [PubMed]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254-65. [Crossref] [PubMed]
- Hagemann T, Lawrence T, McNeish I, et al. “Re-educating” tumor-associated macrophages by targeting NF-kB. J Exp Med 2008;205:1261-8. [Crossref] [PubMed]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889-96. [Crossref] [PubMed]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014;41:21-35. [Crossref] [PubMed]
- Escribese MM, Casas M, Corbi AL. Influence of low oxygen tensions on macrophage polarization. Immunobiology 2012;217:1233-40. [Crossref] [PubMed]
- Leek RD, Lewis CE, Whitehouse R, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996;56:4625-9. [PubMed]
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 2004;114:623-33. [Crossref] [PubMed]
- Joyce JA, Baruch A, Chehade K, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004;5:443-53. [Crossref] [PubMed]
- Shree T, Olson OC, Elie BT, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev 2011;25:2465-79. [Crossref] [PubMed]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015;15:73-86. [Crossref] [PubMed]
- Jiang WG, Sanders AJ, Katoh M, et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol 2015;35 Suppl:S244-75. [Crossref] [PubMed]
- Wu Y, Deng J, Rychahou PG, et al. Stabilization of snail by NF-kappaB is required for inflammation- induced cell migration and invasion. Cancer Cell 2009;15:416-28. [Crossref] [PubMed]
- Pietanza MC, Ladanyi M. Bringing the genomic landscape of small-cell lung cancer into focus. Nat Genet 2012;44:1074-5. [Crossref] [PubMed]
- Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother 2014;15:2385-96. [Crossref] [PubMed]
- Coleman MP, Allemani C. Cancer: the elephant in the room. Lancet 2015;385:1047-8. [Crossref] [PubMed]
- William WN Jr, Glisson BS. Novel strategies for the treatment of small-cell lung carcinoma. Nat Rev Clin Oncol 2011;8:611-9. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- Chen YT, Feng B, Chen LB. Update of research on drug resistance in small cell lung cancer chemotherapy. Asian Pac J Cancer Prev 2012;13:3577-81. [Crossref] [PubMed]
- Murray N. Treatment of small cell lung cancer: the state of the art. Lung Cancer 1997;17:S75-89. [Crossref] [PubMed]
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692-8. [Crossref] [PubMed]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC:beyond traditional treatment approaches. J Thorac Oncol 2013;8:587-98. [Crossref] [PubMed]
- Sgambato A, Casaluce F, Maione P, et al. Medical treatment of small cell lung cancer: state of the art and new development. Expert Opin Pharmacother 2013;14:2019-31. [Crossref] [PubMed]
- Huang R, Wei Y, Hung RJ, et al. Associated Links Among Smoking, Chronic Obstructive Pulmonary Disease, and Small Cell Lung Cancer: A Pooled Analysis in the International Lung Cancer Consortium. EBioMedicine 2015;2:1677-85. [Crossref] [PubMed]
- Hamilton G, Rath B. Smoking, inflammation and small cell lung cancer: recent developments. Wien Med Wochenschr 2015;165:379-86. [Crossref] [PubMed]
- Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol 2014;5:435. [Crossref] [PubMed]
- Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol 2015;44-46:167-74. [Crossref] [PubMed]
- Létuvé S, Kozhich A, Arouche N, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol 2008;181:5167-73. [Crossref] [PubMed]
- Brown V, Elborn JS, Bradley J, et al. Dysregulated apoptosis and NFkappaB expression in COPD subjects. Respir Res 2009;10:24. [Crossref] [PubMed]
- Samadi AK, Bilsland A, Georgakilas AG, et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin Cancer Biol 2015;35 Suppl:S151-84. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-67. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013;13:233-45. [Crossref] [PubMed]
- Young RP, Hopkins RJ. How the genetics of lung cancer may overlap with COPD. Respirology 2011;16:1047-55. [Crossref] [PubMed]
- Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des 2012;18:3831-52. [Crossref] [PubMed]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention:promise, perils and pharmacogenetics. Nat Rev Cancer 2006;6:130-40. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther 2010;87:401-6. [Crossref] [PubMed]
- Rolfo C, Castiglia M, Hong D, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta 2014;1846:539-46.
- Yu N, Zhou J, Cui F, et al. Circulating tumor cells in lung cancer: detection methods and clinical applications. Lung 2015;193:157-71. [Crossref] [PubMed]
- Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol 2012;7:512-9. [Crossref] [PubMed]
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [Crossref] [PubMed]
- Normanno N, Rossi A, Morabito A, et al. Prognostic value of circulating tumor cells' reduction in patients with extensive small-cell lung cancer. Lung Cancer 2014;85:314-9. [Crossref] [PubMed]
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [Crossref] [PubMed]
- Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016;529:298-306. [Crossref] [PubMed]
- Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res 1975;35:512-6. [PubMed]
- Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol 2016;10:395-407. [Crossref] [PubMed]
- Molnar B, Ladanyi A, Tanko L, et al. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res 2001;7:4080-5. [PubMed]
- Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 2004;10:8152-62. [Crossref] [PubMed]
- Krebs MG, Hou JM, Ward TH, et al. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol 2010;2:351-65. [Crossref] [PubMed]
- Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am 2001;10:243-55. [PubMed]
- Sidani M, Wyckoff J, Xue C, et al. Probing the microenvironment of mammary tumors using multiphoton microscopy. J Mammary Gland Biol Neoplasia 2006;11:151-63. [Crossref] [PubMed]
- Mego M, Gao H, Cohen EN, et al. Circulating Tumor Cells (CTC) are Associated with Defects in Adaptive Immunity in Patients with Inflammatory Breast Cancer. J Cancer 2016;7:1095-104. [Crossref] [PubMed]
- Mego M, Giordano A, De Giorgi U, et al. Circulating tumor cells in newly diagnosed inflammatory breast cancer. Breast Cancer Res 2015;17:2. [Crossref] [PubMed]
- Mohamed MM, Al-Raawi D, Sabet SF, et al. Inflammatory breast cancer: New factors contribute to disease etiology: A review. J Adv Res 2014;5:525-36. [Crossref] [PubMed]
- Cayrefourcq L, Mazard T, Joosse S, et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 2015;75:892-901. [Crossref] [PubMed]
- Yu M, Bardia A, Aceto N, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014;345:216-20. [Crossref] [PubMed]
- Medrek C, Pontén F, Jirström K, et al. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012;12:306. [Crossref] [PubMed]
- Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett 2013;332:3-10. [Crossref] [PubMed]
- Rittling SR. Osteopontin in macrophage function. Expert Rev Mol Med 2011;13:e15. [Crossref] [PubMed]
- Rao G, Du L, Chen Q. Osteopontin, a possible modulator of cancer stem cells and their malignant niche. Oncoimmunology 2013;2:e24169. [Crossref] [PubMed]
- Li Y, Sun BS, Pei B, et al. Osteopontin-expressing macrophages in non-small cell lung cancer predict survival. Ann Thorac Surg 2015;99:1140-8. [Crossref] [PubMed]
- Guldur ME, Kibar Y, Deniz H, et al. Comparison of osteopontin, beta-catenin and hnRNP B1 expression in lung carcinomas. Pathol Oncol Res 2010;16:55-9. [Crossref] [PubMed]
- Zhang J, Takahashi K, Takahashi F, et al. Differential osteopontin expression in lung cancer. Cancer Lett 2001;171:215-22. [Crossref] [PubMed]
- Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 2000;6:3282-9. [PubMed]
- Ishii M, Wen H, Corsa CA, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 2009;114:3244-54. [Crossref] [PubMed]
- Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 2011;73:479-501. [Crossref] [PubMed]
- Hamilton G, Rath B, Burghuber O. Chitinase-3-like-1/YKL-40 as marker of circulating tumor cells. Transl Lung Cancer Res 2015;4:287-91. [PubMed]
- Fleischer J, Grage-Griebenow E, Kasper B, et al. Platelet factor 4 inhibits proliferation and cytokine release of activated human T cells. J Immunol 2002;169:770-7. [Crossref] [PubMed]
- Gleissner CA. Macrophage Phenotype Modulation by CXCL4 in Atherosclerosis. Front Physiol 2012;3:1. [Crossref] [PubMed]
- Scheuerer B, Ernst M, Dürrbaum-Landmann I, et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000;95:1158-66. [PubMed]
- White RT, Damm D, Hancock N, et al. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem 1992;267:9210-3. [PubMed]
- Rutkowski MJ, Sughrue ME, Kane AJ, et al. Cancer and the complement cascade. Mol Cancer Res 2010;8:1453-65. [Crossref] [PubMed]
- Sayegh ET, Bloch O, Parsa AT. Complement anaphylatoxins as immune regulators in cancer. Cancer Med 2014;3:747-58. [Crossref] [PubMed]
- Malik S, Fu L, Juras DJ, et al. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci 2013;50:1-22. [Crossref] [PubMed]
- Ding G, Fang J, Tong S, et al. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 2015;75:957-68. [Crossref] [PubMed]
- Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-β family cytokine growth/differentiation factor-15/ macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev 2013;24:373-84. [Crossref] [PubMed]
- Baay M, Brouwer A, Pauwels P, et al. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol 2011;2011:565187.
- Coffman FD. Chitinase 3-like-1 (CHI3l1): A putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci 2008;45:531-62. [Crossref] [PubMed]
- Ma B, Herzog EL, Lee CG, et al. Role of chitinase 3-like-1 and semaphorin 7a in pulmonary melanoma metastasis. Cancer Res 2015;75:487-96. [Crossref] [PubMed]
- Schimpl M, Rush CL, Betou M, et al. Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem J 2012;446:149-57. [Crossref] [PubMed]
- Lee CG, Dela Cruz CS, Ma B, et al. Chitinase-like proteins in lung injury, repair, and metastasis. Proc Am Thorac Soc 2012;9:57-61. [Crossref] [PubMed]
- Tang H, Sun Y, Shi Z, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-κB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol 2013;190:438-46. [Crossref] [PubMed]
- Kzhyshkowska J, Yin S, Liu T, et al. Role of chitinase-like proteins in cancer. Biol Chem 2016;397:231-47. [Crossref] [PubMed]
- Wang J, Sheng Z, Yang W, et al. Elevated Serum Concentration of Chitinase 3-Like 1 is an Independent Prognostic Biomarker for Poor Survival in Lung Cancer Patients. Cell Physiol Biochem 2016;38:461-8. [Crossref] [PubMed]
- Xu CH, Yu LK, Hao KK. Serum YKL-40 level is associated with the chemotherapy response and prognosis of patients with small cell lung cancer. PLoS One 2014;9:e96384. [Crossref] [PubMed]
- Cho SJ, Weiden MD, Lee CG. Chitotriosidase in the Pathogenesis of Inflammation, Interstitial Lung Diseases and COPD. Allergy Asthma Immunol Res 2015;7:14-21. [Crossref] [PubMed]
- Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res 2013;57:99-105. [Crossref] [PubMed]
- Junker N, Johansen JS, Andersen CB, et al. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer 2005;48:223-31. [Crossref] [PubMed]
- Kawada M, Seno H, Kanda K, et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene 2012;31:3111-23. [Crossref] [PubMed]
- Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol Med 2015;7:1-11. [Crossref] [PubMed]
- Francescone RA, Scully S, Faibish M, et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem 2011;286:15332-43. [Crossref] [PubMed]
- Nam KS, Shon YH. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J Microbiol Biotechnol 2009;19:629-33. [PubMed]
- Low D, Subramaniam R, Lin L, et al. Chitinase 3-like 1 induces survival and proliferation of intestinal epithelial cells during chronic inflammation and colitis-associated cancer by regulating S100A9. Oncotarget 2015;6:36535-50. [PubMed]
- He CH, Lee CG, Dela Cruz CS, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep 2013;4:830-41. [Crossref] [PubMed]
- Faibish M, Francescone R, Bentley B, et al. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther 2011;10:742-51. [Crossref] [PubMed]
- Tinhofer I, Saki M, Niehr F, et al. Cancer stem cell characteristics of circulating tumor cells. Int J Radiat Biol 2014;90:622-7. [Crossref] [PubMed]
- Hamilton G, Rath B, Holzer S, et al. Second-line therapy for small cell lung cancer: exploring the potential role of circulating tumor cells. Transl Lung Cancer Res 2016;5:71-7. [PubMed]
- Hamilton G, Hochmair M, Rath B, et al. Small cell lung cancer: Circulating tumor cells of extended stage patients express a mesenchymal-epithelial transition phenotype. Cell Adh Migr 2016;10:360-7. [Crossref] [PubMed]
- Hamilton G, Moser D, Hochmair M. Metastasis: Circulating Tumor Cells in Small Cell Lung Cancer. Trends in Cancer 2016;2:159-60. [Crossref] [PubMed]
- De Palma M, Lewis CE. Cancer: Macrophages limit chemotherapy. Nature 2011;472:303-4. [Crossref] [PubMed]
- Recklies AD, Ling H, White C, et al. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 2005;280:41213-21. [Crossref] [PubMed]
- Hamilton G, Rath B, Ulsperger E. How to target small cell lung cancer. Oncoscience 2015;2:684-92. [Crossref] [PubMed]
- Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res 2014;2:823-30. [Crossref] [PubMed]
- Fan Y, Mao R, Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013;4:176-85. [Crossref] [PubMed]
- Groner B, Lucks P, Borghouts C. The function of Stat3 in tumor cells and their microenvironment. Semin Cell Dev Biol 2008;19:341-50. [Crossref] [PubMed]
- Spitzner M, Ebner R, Wolff HA, et al. STAT3: A Novel Molecular Mediator of Resistance to Chemoradiotherapy. Cancers (Basel) 2014;6:1986-2011. [Crossref] [PubMed]
- Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014;14:736-46. [Crossref] [PubMed]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798-809. [Crossref] [PubMed]
- Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749-59. [Crossref] [PubMed]
- Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta 2010;1805:167-80.
- Mabuchi S, Ohmichi M, Nishio Y, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem 2004;279:23477-85. [Crossref] [PubMed]
- Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res 2012;4:376-89. [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [Crossref] [PubMed]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Paterlini-Bréchot P. Circulating Tumor Cells: Who is the Killer? Cancer Microenviron 2014;7:161-76. [Crossref] [PubMed]


