Towards optimal pathologic staging of resectable non-small cell lung cancer
Accurate staging is essential to the appropriate treatment of cancer. After histologic confirmation of a diagnosis of lung cancer come the questions: ‘what is the prognosis?’, ‘what are the best treatment options?’, ‘how likely is treatment to be successful?’, ‘will chemotherapy be necessary?’ The answer to each of these questions requires knowledge of the stage of the cancer. The tumor, node, and metastasis (TNM) system, our current means of staging lung cancer, serves many functions. It is the language with which we communicate the extent of a patient’s cancer across time and space, provides prognostic information, guides selection among treatment alternatives, and is a key aspect in selecting patients for clinical trials.
Advances in technology have improved the accuracy of clinical staging. Clinical staging incorporates all non-invasive radiologic tests such as computerized tomography (CT), positron emission tomography (PET), magnetic resonance imaging, and bone scans (1,2). In the surgical resection population, in which distant metastasis has usually been ruled out, the most difficult staging problem is the accurate determination of nodal metastasis status. Radiologic determination of the size and extent of the primary tumor is fairly accurate, although delineating the T3-T4 border, i.e., determining whether a tumor that seems to extend to major mediastinal structures is actually invasive (T4) or merely abutting (T3), can sometimes only be resolved at thoracotomy. However, nodal status is the most important determinant of survival in the lung cancer patient who does not have distant metastatic disease, and the question of lymph node metastasis is less easily resolved by radiologic tests (1,3). Invasive clinical staging of mediastinal lymph nodes may be accomplished by transbronchial needle aspiration, endobronchial ultrasound guidance, endoscopic ultrasound guidance, mediastinoscopy, video-assisted mediastinal lymphadenectomy, transcervical extended mediastinal lymphadenectomy or video-assisted thoracoscopy (2,4,5).
However, clinical staging tests have their sensitivity, specificity and accuracy limitations. The positive predictive value (PPV) for CT ranges from 0.16 to 0.88 and the negative predictive value (NPV) ranges from 0.54-0.83 (1). Specifically, normal sized lymph nodes by CT criteria may harbor metastatic disease and enlarged lymph nodes may be enlarged because of benign processes such as postobstructive pneumonia, histoplasmosis, and sarcoidosis. The likelihood of an enlarged mediastinal node being histologically positive is only 60% whereas 20% of normal sized nodes may harbor metastasis (6). Similarly, PET-positive nodes may have increased metabolic activity because of an inflammatory process whereas histologically positive nodes may be negative on PET because of low metabolic activity or low burden of disease. Although PET performs better than CT, with a PPV ranging from 0.40 to 1.00 and a NPV ranging from 0.71-1.00, the false-negative rate is approximately 20% for normal sized nodes. Conversely, enlarged nodes that are PET positive are falsely positive 15-25% of the time (1). Invasive tests have limits imposed by the reach of the instrument and the degree of effort applied by the operator, or what Frank Detterbeck has described as the ‘thoroughness of execution’ (7).
Recent studies have demonstrated the value of combining clinical staging tests in the pre-operative work up of patients (8,9). For this reason current staging guidelines, including Cancer Care Ontario’s Program in Evidence Based Care Practice Guidelines, recommend invasive mediastinal staging in the presence of either enlarged nodes on CT or “hot” nodes on PET to rule out false-positive imaging tests. These guidelines also recommend invasive mediastinal staging even with a negative CT and PET for high risk tumors (defined as central, large, T3/T4, or adenocarcinoma) (10).
For all the advances in clinical staging options, the most accurate determination of stage in patients who are able to undergo surgical resection comes from examination of the resection material obtained at thoracotomy (pathologic staging) (11). Comparison of the 5-year survival rates in groups of patients who are staged by clinical and pathologic means reveals a 5-23% higher survival in patients with pathologic stage I, II, and IIIA over those with the identical clinical stage (Table 1) (12). This difference is independent of the combination of descriptors used to assign aggregate stage, and is probably partly explained by the ‘Will Rogers phenomenon’, in which improved staging accuracy leads to more accurate assignment of low risk patients into low risk groups and upstaging of seemingly low risk patients with subtle metastatic disease into higher risk categories, thereby improving the aggregate outcomes of the higher risk cohorts (13). Pathologic staging is therefore our most accurate prognostic tool in lung cancer.
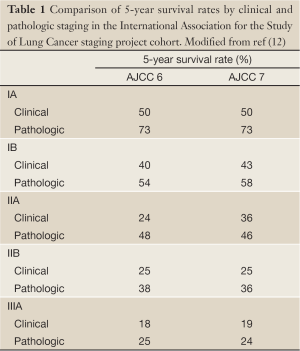
Full Table
However, current pathologic staging of lung cancer remains insufficiently discriminatory of future patient outcomes. For example, the 5-year survival of patients with resected stage IA non-small cell lung cancer (NSCLC) is 73%, meaning the mortality rate of the lowest risk cohort is 27% (Table 1) (12). Although lymph node metastasis is our most powerful prognostic determinant in the surgical resection population, the 5-year survival of patients with pathologic N0 NSCLC is 56%, meaning that 44% of patients with apparently low risk disease die within 5 years (14). Are these poor results solely due to the biologic aggressiveness of lung cancer (or the frailty of the lung cancer patient), or do they reflect other problems such as limitations of the TNM staging system as a prognostic tool, or, very importantly-because of the opportunity for corrective intervention-poor application of the prognostic tool?
Determining the stage-relevant characteristics of the primary tumor (its size and extent of direct invasion) is relatively straightforward for the pathologist. In the surgical resection population, distant metastasis usually being inevident, the most important pathologic staging problem is determining lymph node metastasis status. This requires the collaborative efforts of the surgeon (to retrieve the hilar and mediastinal lymph nodes, and to accurately communicate the provenance of all lymph node specimens to the pathologist for accurate mapping) and the pathologist (to examine all lymph nodes in the resection specimen, both those directly provided by the surgeon and those indirectly provided within the lung resection material). There is compelling evidence that this collaborative effort frequently breaks down, to the detriment of patients.
At one extreme, 13% of all curative-intent resections (and 18% of resections for ‘node-negative disease’) have no lymph nodes examined (15). The survival of patients with pathologically ambiguous nodal stage (pNX) approximates very closely to that of patients with pN1, not pN0 disease (when pN0 is defined as actually having at least one examined lymph node), suggesting that a significant proportion have missed lymph node metastasis (15). Secondly, 40-50% of all curative lung cancer resections in large North American databases have no mediastinal lymph nodes examined (16,17). Indeed, 63% of resections for mediastinal node negative (pN0 or pN1) disease in the US Surveillance, Epidemiology, and End Results (SEER) database from 1998 to 2009 had no mediastinal lymph nodes examined, leading to a 14% survival deficit (17). To put this survival impact in perspective, the estimated absolute survival benefit of post-operative adjuvant chemotherapy is about 5.4% (18). This problem is not unique to the US (19).
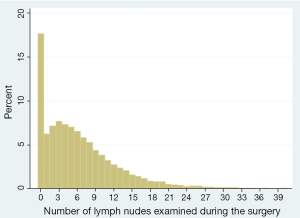
Furthermore, and more subtly, most patients with pathologic N0 disease cluster at the low end of the total lymph node number spectrum, with a median lymph node count of 6 in the US (Figure 1) (20). Patients with fewer than 6 lymph nodes have a significantly worse survival than matched patients with greater than 6 lymph nodes despite ostensibly having the same pathologic stage (21,22). Hence the recommendation in the 7th edition of the AJCC/UICC staging guidelines for examination of at least 6 lymph nodes and 3 nodal stations (23). However, this recommendation is probably insufficiently stringent because of evidence of sequential improvement in survival of patients with pathologic N0 disease with increasing number of lymph nodes examined, with the optimal number being ‘greater than 10’ and possibly as high as 18 to 21 (20,24-26). It is therefore unsettling that fewer than 15% of all pN0 lung cancer resections in large US databases have examination of greater than 10 lymph nodes. Even in patients with lymph node metastasis, there is prognostic value to the number of lymph nodes examined, both in helping determine the absolute number of lymph nodes with metastasis and in determining the ratio of positive and negative lymph nodes (27-32).
The etiology of suboptimal nodal examination has been the subject of recent investigation. Conceptually, it appears reasonable to separate the origin of the problem into three sites: events during the surgical operation (such as the hilar and mediastinal lymph node harvest), events during the transfer of specimens from the operating room to the pathology laboratory, and events during the pathology examination. Clearly, when surgeons do not harvest hilar and mediastinal lymph nodes, pathologists have no access to material for a thorough staging examination. Therefore, the solution to the problem of non-examination of mediastinal lymph nodes might be best achieved by focusing on intraoperative events. However, surgeons frequently complain that the specimens they submit are not completely examined. This assertion may be supported by ‘before and after’ intervention studies in which use of pre-labeled specimen collection kits improves the quality of pathologic staging, with a reversion to pre-intervention levels during the intervention phase in cases when the kit is inadvertently unavailable (33).
It therefore seems plausible that the communication between surgeons and pathologists during the transfer of specimens needs to be improved. Solutions might range from prevention of specimen loss in transit (34), to improved labeling of specimens in order to improve the ability of pathologists to determine the source and nature of submitted materials (35). Both of these factors (loss of specimens in transit, and inadequate specimen labeling) may impair the pathologic examination and lymph node mapping. The foregoing notwithstanding, the gross dissection of lung resection specimens for intrapulmonary lymph nodes may be an opportunity for pathology-centered quality improvement (36). For example, 10% of patients with one or more lymph nodes examined have no N1 lymph nodes, meaning that but for the mediastinal lymph nodes provided by the surgeon, there would have been no nodes examined in the resection specimen (37). Pathologists not infrequently omit the pathologic nodal stage in the report summary, or make errors in stage attribution, such as labeling N1 disease as N2 and vice-versa. This combination occurred in 33% of pathology reports in one city-wide audit of lung resection pathology reports (38). The very existence of the 12-18% pNX population is the clearest illustration of the possibility of concurrent glitches in intraoperative and pathology processes.
All of this naturally raises the question: what is the optimal surgical resection and pathologic staging procedure? We shall not engage the debate about the extent of resection and whether, or not, sublobar resection is oncologically sound in lobectomy candidates, a topic that remains the subject of ongoing clinical trials in North America (Cancer and Leukemia Group B 140503, clinicaltrials.gov #00499330) and Japan (Japan Clinical Oncology Group 0802/West Japan Oncology Group 4607L); Nor shall we address the looming controversy about the appropriateness of lobar resection in patients with low grade lesions such as adenocarcinoma in-situ, minimally invasive adenocarcinoma and ground glass opacity (39); Nor shall we discuss the definition of an oncologically complete resection for lung cancer, a topic of much interest which has been provocatively addressed in the recent past (40). Our focus is primarily on the lymph node staging problem.
The optimal surgical lymph node staging procedure has been partially clarified by the landmark American College of Surgery Oncology Group Z0030 trial which compared the long-term survival of patients with clinical T1-2, N0-1 NSCLC who underwent a fastidious, pre-specified systematic sampling procedure versus a more extensive mediastinal nodal dissection (41). Although 4% of patients in the extensive dissection arm had lymph node metastasis that had been missed by the systematic sampling procedure, there was no difference in recurrence free- or overall survival between the two groups. Early data analyses from this trial established the safety of mediastinal lymph node dissection in both academic and community care settings (42). It also revealed that surgeons’ attention to the mediastinal lymph node harvest procedure provides a much higher lymph node yield than usually obtained—a median of 18 additional lymph nodes were collected in the mediastinal lymph node dissection arm (two-thirds of which were N2 lymph nodes), 6 or more nodes were examined from a minimum of 3 nodal stations in >99% of patients, and a minimum of 10 lymph nodes were examined from at least 3 nodal stations in 90% of patients (43). Most importantly, ACOSOG Z0030 definitively established the adequacy of systematic sampling as an oncologically sound mediastinal lymph node staging procedure in patients with relatively low risk early stage NSCLC and is now oft-cited in support of a pathologic staging strategy short of formal mediastinal nodal dissection (44).
However, it is important that we interpret Z0030 in the right context. First, the eligibility criteria specifically excluded patients with cT3 and T4 tumors, and those with hilar or mediastinal lymph node metastasis on frozen section analysis of the lymph nodes collected after the rigorous systematic nodal sampling procedure. Therefore, the results of this trial must not be misinterpreted as proof of equivalency between the two nodal dissection procedures in higher risk patients, such as those with clinically more advanced disease, because the results may be dissimilar in these patients. Secondly, this trial cannot be cited in support of the idea that noninvasive staging (with CT and PET) is a substitute for surgical mediastinal lymph node staging. It must be emphasized that all patients in Z0030 received a fastidious nodal sampling procedure, which included sampling of lymph nodes from stations 2R, 4R, 7 and 10R for right-sided tumors and stations 5, 6, 7 and 10L for left sided tumors regardless of lymph node size or metabolic activity. The randomization to cessation of further nodal dissection versus complete mediastinal lymph node dissection was performed only after establishment of histologic node negativity in stations 2-10, and the survival analysis included only patients who met the stringent quality criteria for the nodal sampling procedure. Z0030 cannot be used to justify a strategy of either no mediastinal nodal sampling (which is the experience of a large proportion of patients who undergo resection in US databases) (16,17) or random sampling (the experience of the vast majority of all others) (45).
A prior study by Wu et al. corroborates the veracity of the above observations (46). In this study, 532 patients with clinical stage I, II or III NSCLC were randomized to either mediastinal lymph node dissection or to a nodal sampling procedure that was much less thorough than Z0030, requiring hilar nodal dissection, routine harvesting of station 7 and inspection of stations 1-9 with only removal of ‘nodes with suspected cancer metastasis (diameter >1 cm or hard)’. They reported improved survival in favor of node dissection with a median survival of 43 months compared to 32 months for sampling (P=0.0001). In contrast to Z0030, patients had no cytological or histological assessment of lymph nodes prior to randomization and resection, suggesting that if pre-resection systematic lymph node sampling has not been performed, survival is improved by mediastinal lymph node dissection (46).
In one community-based series, only 8% of patients who had lung resection over the course of a 4-year time span met criteria for a less stringent definition of systematic sampling than was performed in Z0030 (45). This study highlighted the loose use of terminology by surgeons: in the 45% of resections in which the surgeon reported having performed a ‘mediastinal lymph node dissection’, objective review of the pathology report suggested that none met the Z0030 mediastinal nodal dissection criteria, 9% were better classified as systematic sampling, 50% had random sampling and 42% had no mediastinal lymph nodes examined. It would be an unfortunate misunderstanding of the state of the evidence for the results of Z0030 to be used to justify such practice.
A less obvious side-bar to the discordance between surgeon procedure claims and the results of pathology report-based audits of the quality of nodal examination is the contribution of pathology practice. Despite the consensus statement that pathologists should ‘examine all lymph nodes in the lung resection specimen’ (47), re-examination of lung resection specimens after completion of routine pathology examination reveals that 137% more intrapulmonary lymph nodes (and 165% more lymph nodes with metastasis) can be retrieved from discarded lung specimens than the number retrieved during the routine examination (36). Indeed, up to 12% of patients said to have pN0 disease on routine examination, had identifiable lymph node metastasis by hematoxylin and eosin staining of discarded lymph nodes. Using fastidious intrapulmonary nodal retrieval procedures, a median of 11 N1 lymph nodes were retrieved from lobar lung resection specimens, up from a pre-intervention median of 3 N1 nodes (36). Interestingly, this is greater than the median of 5 to 6 N1 lymph nodes examined in the ACOSOG Z0030 trial, even though per study protocol surgeons helped retrieve nodes from stations 10-13 (43). This suggests that the opportunity for quality improvement in routine pathology examination of lung resection specimens exists across different types of institutions. This opportunity might be greater in routine practice because of the expectation most surgeons have that nodes within the resection specimen would be retrieved by gross dissection in the pathology laboratory.
It is incumbent on the surgeon to provide adequate N2 nodes through systematic sampling or mediastinal lymph node dissection, but also to harvest N1 nodes including stations 10 and 11. Recent data demonstrated significant upstaging with respect to N1 nodes in open compared to VATS lobectomy suggesting that surgeons were not harvesting the hilar zone nodes when performing VATS lobectomy (48). Clearly, the pathologist cannot examine nodes that are left in the chest. Optimal pathologic nodal staging requires the collaborative actions of surgeons, members of the operating room team, specimen handlers, the pathology laboratory team and the pathologist. A chain of actions is required for optimal pathologic staging of curatively resected lung cancer. Like all chains, it is only as strong as its weakest link. Effective interventions to correct the prevailing quality deficit in staging must encompass the full spectrum of potential sites of quality breakdown, from the surgical operation to the posting of the final pathology report.
Interventions in which pre-labeled specimen collection kits have been combined with fastidious gross dissection of the lung resection specimen demonstrate early promise in rectifying the quality deficit. Studies of these interventions suggest that the proportion of patients found to have nodal metastasis increases significantly, with strong trends towards significant upward aggregate stage migration (49). Unfortunately, these studies do not yet provide data on the survival impact of these quality improvement measures (50-52). Despite the paucity of data on survival impact and cost-effectiveness of these corrective interventions, it seems prudent to narrow or eliminate the quality gap in pathologic nodal staging, given its well-documented adverse impact on patient survival.
It is also important to emphasize that the results of Z0030 should be applied to patients with relatively early clinical stage NSCLC. These results cannot automatically be extrapolated to patients with more advanced disease. In addition, we propose that systematic sampling must be performed at least as rigorously as in Z0030 in order to provide sufficient quality pathologic staging for patients who undergo staging by that strategy. Calling a procedure ‘systematic sampling’ or ‘mediastinal lymph node dissection’ does not necessarily make it so. The definitions must be based on the actual lymph nodes retrieved from specific stations, all of which must be clearly labeled for, and examined by, the pathologist.
In conclusion, there is a great need to heighten general awareness of the prevalence and severity of the quality gap between optimal, recommended, nodal staging of resectable lung cancer, and actual practice. This awareness campaign must be sponsored and supported by all the clinical professional groups with influence over the problem, including associations of surgeons, pathologists, medical oncologists and radiation oncologists, and their various guidelines-making bodies. Research into the evaluation and implementation of corrective solutions must be supported by funding agencies, in order to provide clear evidence with which healthcare policymakers can develop incentives that will ultimately facilitate the elimination of this major quality of care deficit.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S.
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-220S.
- Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg 2003;238:180-8. [PubMed]
- Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139:879-92. [PubMed]
- Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest 2010;137:436-42. [PubMed]
- Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [PubMed]
- Darling G, Dickie J, Malthaner R, et al. Invasive mediastinal staging of non small cell lung cancer. Available online: http://www.cancercare.on.ca/toolbox/qualityguidelines/clin-program/surgery-ebs/t. Accessed on 6.17.13.
- D’Cunha J, Herndon JE 2nd, Herzan DL, et al. Poor correspondence between clinical and pathologic staging in stage 1 non-small cell lung cancer: results from CALGB 9761, a prospective trial. Lung Cancer 2005;48:241-6. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985;312:1604-8. [PubMed]
- Rusch VW, Crowley J, Giroux DJ, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:603-12.
- Osarogiagbon RU, Yu X. Nonexamination of Lymph Nodes and Survival After Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2013;96:1178-89. [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [PubMed]
- Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol 2012;7:1798-806. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Verhagen AF, Schoenmakers MC, Barendregt W, et al. Completeness of lung cancer surgery: is mediastinal dissection common practice? Eur J Cardiothorac Surg 2012;41:834-8. [PubMed]
- Osarogiagbon RU, Ogbata OU, Yu X. The number of lymph nodes associated with maximal reduction of long term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. In press, accepted 9.16.13.
- Gajra A, Newman N, Gamble GP, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [PubMed]
- Osarogiagbon RU, Yu X. Comparative survival of resected node-negative non-small cell lung cancer with and without lymph node examination in the SEER database. J Thorac Oncol 2012;7:abstr 131.
- Goldstraw P. eds. International Association for the Study of Lung Cancer Staging Handbook in Thoracic Oncology. Orange Park, FL: Editorial Rx Press, 2009.
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [PubMed]
- Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009;115:851-8. [PubMed]
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5. [PubMed]
- Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg 2008;85:211-5. [PubMed]
- Jonnalagadda S, Smith C, Mhango G, et al. The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest 2011;140:433-40. [PubMed]
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [PubMed]
- Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724-31. [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20. [PubMed]
- Osarogiagbon RU, Miller LE, Ramirez RA, et al. Use of a surgical specimen-collection kit to improve mediastinal lymph-node examination of resectable lung cancer. J Thorac Oncol 2012;7:1276-82. [PubMed]
- Makary MA, Epstein J, Pronovost PJ, et al. Surgical specimen identification errors: a new measure of quality in surgical care. Surgery 2007;141:450-5. [PubMed]
- Molnar TF. A new device for the identification of lymph nodes at lung cancer surgery. Eur J Cardiothorac Surg 2007;31:311-2. [PubMed]
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823-8. [PubMed]
- Allen JW, Farooq A, O’Brien TF, et al. Quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer 2011;117:134-42. [PubMed]
- Farooq A, Osarogiagbon RU, Allen JW, et al. Accuracy and comprehensiveness of pathology reportage after lung cancer resection. J Clin Oncol 2009;27:abstr 6523.
- Nakao M, Yoshida J, Goto K, et al. Long-term outcomes of 50 cases of limited-resection trial for pulmonary ground-glass opacity nodules. J Thorac Oncol 2012;7:1563-6. [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy: results of the randomized, prospective American College of Surgeons Oncology Group Z0030 trial. Chest 2011;139:1124-9. [PubMed]
- Murthy SC. Less is more… (more or less…). J Thorac Cardiovasc Surg 2011;141:670-2. [PubMed]
- Osarogiagbon RU, Allen JW, Farooq A, et al. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol 2012;7:390-6. [PubMed]
- Yl Wu. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [PubMed]
- Association of Directors of Anatomic and Surgical Pathology. Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol 2001;115:799-801. [PubMed]
- Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94:347-53; discussion 353. [PubMed]
- Osarogiagbon RU, Ramirez RA, Wang CG, et al. Dual intervention to improve pathologic staging of resectable lung cancer. Ann Thorac Surg 2013. [Epub ahead of print]. [PubMed]
- Brzezniak C, Giaccone G. Intrapulmonary lymph node retrieval: unclear benefit for aggressive pathologic dissection. Transl Lung Cancer Res 2012;1:230-3.
- Osarogiagbon RU, Miller LE, Wang CG, et al. Response to editorial titled ‘Intrapulmonary lymph node retrieval: unclear benefit for aggressive pathologic dissection’. Transl Lung Cancer Res 2013;2:E33-E36.
- Riquet M, Mordant P. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. Transl Lung Cancer Res 2013;2:1-2.


