Post-operative radiation therapy
Introduction
Even after complete resection of operable non-small cell lung cancer (NSCLC), patients are at high risk of recurrence (1). This risk of relapse is both distant and local, so that adjuvant chemotherapy as well as radiotherapy have been evaluated in randomized, though often underpowered, trials.
Concerning adjuvant chemotherapy, the results of the first published meta-analysis were updated in 2010 (2) including a total of 8,447 patients with both data from the old trials, and from all recent trials, showing an absolute increase in survival of 4% at 5 years (from 60% to 64%, P<0.0001). The beneficial effect of adjuvant chemotherapy was also observed in the LACE meta-analysis, which only included trials with cisplatin-based regimen (3), showing an absolute survival benefit at five years of 5.4% (P=0.005). After complete resection, adjuvant chemotherapy is now a standard of care for stage II and III NSCLC patients, even for elderly patients (4), but is controversial in stage I patients (5). Nonetheless, even among these patients, local control remains an important issue as 20-40% of patients will suffer from loco-regional relapse.
Concerning post-operative radiotherapy (PORT), it has been evaluated for decades, and, despite several trials and meta-analysis, it is still debated.
PORT through the prism of evidence-based medicine
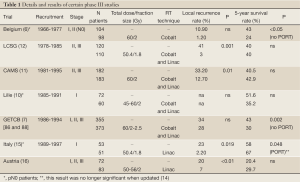
The PORT meta-analysis which initially included 9 randomized trials (6-12) is a landmark study published in 1998 (13) and updated in 2005 (14) with a 10th study (15). The conclusions of this meta-analysis are well known among clinicians: PORT is detrimental to patients with early stage (I or II), whereas for those with N2 disease there was no significant adverse effect. The details of the 10 trials are much less known than the conclusions of the meta-analysis, however, these details are of paramount importance to better understand the possible role of PORT in the N2 subgroup of patients which is not clear as shown in Tables 1,2. It should be outlined that none of these trials used adjuvant chemotherapy which is now a standard (2,3).
Full Table
Three randomised trials were dedicated to early stage (pN0) patients. The first trial was performed by Van Houtte et al. (6) and included 175 N0 patients from 1966 to 1977. PORT was delivered with a cobalt 60 unit (Co). The 5-year survival rate was respectively 24% in the RT arm and 43% in the control arm. The deleterious effect of RT was even more pronounced after pneumonectomy with a survival rate of 16% with PORT and 43% in the control arm. A study performed a decade later, by the same team (17), has highlighted the potential benefit of modern facilities (linear accelerator and computed tomography-based treatment planning): the 5-year survival rate was only 8% among patients treated with Co, whereas it was 30% in patients treated with more modern radiotherapy. The second trial dedicated to pN0 patients is the study of Lafitte et al. (10) which found no significant difference in overall survival or local control between surgery and PORT versus surgery alone. The authors pointed out that the main pattern of relapse was distant recurrence and that systemic adjuvant therapy should be considered. The third study performed by Trodella et al., also focused on pN0 patients (15). PORT was delivered at the dose of 50.4 Gy in 28 fractions of 1.8 Gy, using modern facilities as described above. The first results published in 2002 showed a positive trend in overall 5-year survival in favor of PORT (67% versus 58% in the control arm, P=0.046), but this trend was not confirmed when data were reanalyzed for the update of PORT meta-analysis (14). Even if this trend was unconfirmed, the authors highlighted, that the treatment fields were very limited, and that there was no detrimental effect related to PORT in this trial which used modern radiotherapy. However, it is now generally considered that such pN0 patients are more at risk of distant failure than local failure.
Three randomized studies included stage II and III, or pN1 and pN2 patients, excluding thus pN0 patients. In the randomized study conducted by the Lung Cancer Study Group (LCSG) (12), 230 patients with stage II or III resected squamous cell carcinoma were enrolled. There was no significant difference in overall survival, with a 5-year survival rate of about 40% in both arms, although PORT reduced significantly the rate of local recurrence (1% with PORT and 41% in the control arm, P<0.001). Moreover, subgroup analysis suggested that disease free survival (DFS) could be prolonged by PORT for N2 patients. The study of the Medical Research Council (MRC) (9) had a similar design to the LCSG trial, but it also included patients with adenocarcinoma. The results were quite similar with better local control that did not translate into a significant overall survival benefit. Once again, subgroup analysis revealed a trend for better overall survival in N2 patients. A phase III Chinese trial involving 366 N1 or N2 resected patients, came to the same conclusions: they found a lower rate of local recurrence (12.7% with PORT and 33.2% in the control arm, P=0.01) with no impact on survival (5-year survival rate was respectively 42.9% with PORT and 40.5% in the control arm, P=0.56).
Finally, the study of Dautzenberg et al. (7) which is the largest trial included in the meta-analysis on PORT, included 728 patients: 221 with stage I, 180 patients with stage II and 327 patients with stage III. The authors observed a detrimental effect of PORT on survival: 5-year overall survival rate was 30% with PORT versus 43% in the control arm (P=0.002). Once again for N2 patients, there was a trend in favor of PORT in decreasing loco-regional relapse. The excess of deaths among patients treated with PORT was due to a high incidence of cardiac and respiratory complications (such as cardiorespiratory failure, radiation pneumonitis, and massive haemoptysis). These non-cancer-related deaths seemed correlated with fractionation: they were much more frequent among patients who had received a daily fraction of 2 Gy or more (26% with daily fraction >2 Gy versus 16-18% in case of daily fraction ≤2 Gy). In the Mayer study (16), which was not included in the meta-analysis, 155 completely resected patients with T1-3 N0-2 NSCLC were randomly assigned to observation or PORT. The results (16) were similar to those of the LCSG (12), CAMS (11) or MRC (9): a significant increase of local control could be observed among patients who had PORT but with no impact in overall survival.
In the early 90s, for operable patients with small N2 nodal involvement, surgery and PORT was considered as a standard of care, before publication of several studies and meta-analyses (18-20) had proven the beneficial effect of adjuvant (or neo-adjuvant) chemotherapy in this group of patients [IALT, JBR, ANITA, LACE, Meta-analyses 2010, 30]. Therefore, the Eastern Cooperative Oncology Group (ECOG) proposed a randomised phase III study which allocated patients who had complete surgery to PORT (50.4 Gy in 1.8 Gy fractions), which was considered as the reference arm, or to chemo-radiotherapy (CPORT: cisplatin and etoposide regimen administered concurrently with PORT) (21). There was no significant difference between the 2 arms, neither in terms of survival (3-year survival rates respectively of 52% with PORT and 50% with CPORT, P=0.56), nor local recurrences (13% with PORT and 12% with CPORT, P=0.84). Interestingly, the authors performed a retrospective analysis in order to compare the impact of a simple systematic sampling versus a complete mediastinal lymph node dissection (22) and found a survival advantage among patients who had a mediastinal lymph node dissection. Nonetheless, the result of this nonrandomized and non-planned comparison should be interpreted with caution. They outline that the modalities of surgery and most importantly nodal exploration are also important to consider, in order to evaluate PORT. Finally in this chapter it seems important to mention that a group of the studies included in the meta-analysis published in 2010, based on individual data from 13 randomised trials (2,660 patients; 63% being stage III) has evaluated the combination of surgery plus PORT which was the control arm versus surgery plus PORT and adjuvant chemotherapy which was the investigational arm (2). An absolute and significant survival benefit of 4% in favor of adjuvant chemotherapy was found (the 5-year survival rates were 29% in the surgery plus PORT and 33% with surgery plus PORT and chemotherapy, P=0.009). This survival benefit was similar to that observed with surgery plus chemotherapy compared to surgery alone. The authors concluded that the benefit of chemotherapy was similar irrespective of whether PORT was added to surgery or not. So adjuvant chemotherapy has become the standard of care, for stages II and III patients. The question would now concern PORT which should be in the investigational arm, whereas the control arm would include surgery plus chemotherapy.
Could an increase of loco-regional control be beneficial to high risk patients?
Four randomised trials including N2 patients [LCSG (12), CAMS (11), MRC (9) and the Austrian trial (16)] found PORT to be associated with a significant decrease of local failure but with no impact on survival. The study of Dautzenberg et al. (7) suggested that PORT could improve local control only in N2 patients. It should be outlined that these studies were performed at an era where staging evaluation did not comprise PET-CT scan and brain imaging, so that several patients included in these trials, especially those with N2 disease, might have been metastatic at the time they were included. Thus, the potential effect of PORT on local control may also have been diluted by the occurrence of distant metastases. As adjuvant chemotherapy is now part of the standard treatment in these patients, PORT needs to be reevaluated in the subgroup of patients who, after a complete staging evaluation with PET-CT and brain imaging, are found to be pN2. The question of PORT may also be valid in high risk patients who have pre-operative chemotherapy whether or not they have nodal downstaging. As shown in a Swiss phase II study which evaluated neo-adjuvant chemotherapy in stage IIIA patients with proven pathological N2 disease, the rate of local relapse can be high as it reached 60% at 5 years (23). Recently, Mauguen et al. (24) have found that disease free survival seemed to be a valid surrogate endpoint for overall survival. This finding also suggests that improving local control and disease free survival might improve survival.
Within the Surveillance, Epidemiology, and End Results (SEER) database (25), 7,465 patients treated from 1988 to 2002 (a time period in which linear accelerators were already common in clinical treatment) were retrospectively analysed. The same conclusions were drawn than those suggested by PORT meta-analysis (13,14): a survival benefit for N2 patients and a detrimental effect on survival for N0 and N1, even if it can be extrapolated that patients were treated with more modern radiotherapy techniques. Among the 840 patients included in the ANITA trial (20), 232 received PORT. Survival of patients with and without PORT in each arm (adjuvant chemotherapy or observation) was well described (26): in univariate analysis, PORT had a detrimental effect on survival, but, in the subgroup of patients with N2 disease, survival was improved with PORT both in the chemotherapy (median survival of 23.8 months without PORT and 47.4 months with PORT) and the observation arm (median survival of 12.7 months without PORT and 22.7 months with PORT). The author thereby advocated that further evaluation of PORT in completely resected pN2 NSCLC should be performed in randomized trials. Scotti et al. have retrospectively reviewed the data of 175 patients with completely resected N2 disease (27). Local failure rates were 15.1% in the PORT group and 32.1% in the no-PORT group (P=0.009), but overall survival was similar in both groups. For these patients treated between 1988 and 2004, radiotherapy has resulted in mild toxicity.
Patients with ipsilateral mediastinal lymph node involvement are a heterogeneous subgroup. From a retrospective study involving 702 patients who underwent surgery in 6 French centers, Andre et al. (28) have used a subclassification taking into account 2 criteria concerning nodal involvement: minimal (mN2: no preoperative detection of N2 disease) or clinical (cN2: enlarge lymph node on CT scan) disease, and single (L1) or multiple (L2) lymph node involvement. The 5-year survival rates for patients treated with primary surgery were dramatically different within subgroups: mN2 L1 (244 patients): 34%, mN2 L2 (78 patients): 11%, cN2 L1 (118 patients): 8% and cN2 L2 (122 patients): 3%. Unfortunately, no data were available concerning the pattern of relapse. For the authors, the poor prognosis of cN2 patients leads to propose multimodality treatment, such as peri-operative (neoadjuvant and adjuvant) chemotherapy and PORT. We have seen that surgery and adjuvant chemotherapy is now the standard of care (2,29), thus, neoadjuvant chemotherapy is the preferred sequence by some clinicians. However, a recent meta-analysis didn’t find any survival difference between pre and post-operative chemotherapy (30). As mediastinal downstaging after induction treatment is a strong and a relevant prognostic factor (31,32), one option is to refer operable patients with cytologically or pathologically proven ipsilateral mediastinal node involvement to surgeons in cases of response to preoperative chemotherapy.
Ichinose et al. were able to retrospectively assess 332 completely resected N2 patients between 1992 and 1993 in Japan (33). Out of these 332 patients, 130 (39.2%) experienced local failure. The number of N2 stations was found to be a prognostic factor for local recurrence. Another Japanese study has retrospectively assessed PORT according the number of lymph node stations involved (34). PORT had no significant effect on overall survival, but significantly improved disease free-survival (by decreasing local recurrence) in patients with multiple N2 involvement. In this subgroup of patients, the 5-year disease-free survival rates were 41% in the PORT group and 5.9% in the non-PORT group. The same concept was tested by Urban et al. who analysed 11,324 patients from the SEER database (35). Their results suggest, once again, that PORT is beneficial to patients with pN2 disease, when the lymph node ratio (number of positive nodes/number of resected nodes) is at least 50% or more.
Is the detrimental effect of PORT still an issue with modern radiotherapy?
All patients randomised in the 11 phase 3 studies evaluating PORT were treated before 2000; some were even included as early as 1966. It must be emphasized that only 3 out of the 11 randomized trials evaluating PORT used exclusively modern radiotherapy, i.e., computed tomography based treatment planning and linear accelerator delivering high energy [(15,16) and the unpublished EORT 08861 trial], and a substantial number of patients enrolled in these studies were treated with Co unit. As shown in Tables 1,2, a worse survival was found in the Belgian (6) and the GETCB studies (7), which use Co, high total dose (60 Gy) and high dose per fraction (>2 Gy). It has been demonstrated that using more “modern” radiotherapy, resulted in lower morbidity than treatment with cobalt unit (17). Moreover, the use of a 2-dimensional technique, instead of CT-based 3 dimensional conformal radiotherapy, leads to an underdosage in the area at risk (36). The total dose, the dose per fraction and the treated volume are also of major concern when considering toxicity. Firstly, the total dose delivered to the majority of patients included in the meta-analysis (6,7,10,11) was as high as 60 Gy, whereas 54 Gy would be sufficient in a prophylactic setting and less harmful (37). Secondly, it is well demonstrated that fractionation schedules with more than 2.5/3 Gy per fraction leads to a higher rate of cardiac (38) and pulmonary (39) injury. Dautzenberg et al. (7) highlighted the detrimental effect of large doses per fraction in PORT. In most studies included in the meta-analysis, the irradiated volume was usually quite large and included most of the mediastinum (both the ipsi and contra lateral side of the mediastinum) and the supraclavicular area. Incidence of nodal involvement derived from surgical series (40) and the pattern of relapse after surgery without PORT (41) may help to define higher risk areas. To further improve the definition of the nodal areas at risk, a CT-based node map, derived from the classification proposed by Mountain and Dresler (42), has been defined (43). However there have been changes concerning the delimitations of nodal stations and introduction of the nodal zone concept in the new TNM classification (44).
Breast cancer provides an interesting model of benefit/risk balance with adjuvant radiotherapy: despite a decreased risk of local recurrence with radiotherapy, no significant impact on overall survival has been proven until the end of the 90s (45,46), due to an increased risk of mortality from ischemic heart disease. With modern radiotherapy, a significant benefit in favor of radiotherapy has finally be highlight (45,46), and it has been shown that the risk of death from heart disease has substantially decreased more contemporary techniques of radiotherapy (47).
For patients suffering from NSCLC, some retrospective data suggest that PORT-related toxicity might have also decreased with time. Rate of death from intercurrent disease (DID) of the patients included in the ECOG study, which evaluated PORT versus CPORT (21), was compared to the expected rate of DID calculated from United States vital statistics (48). The 4-year rate of DID was 12.9% for patients treated in the ECOG study and was not significantly different from the 10.1% 4-year expected rate of DID (P=0.16). Data concerning 6,148 patients treated from 1983 to 1993 were obtained from the SEER program (49): 3,589 received PORT (58%) and 2,559 did not (42%). PORT was significantly associated with an increased risk of death from heart disease. However, this excess of cardiovascular toxicities after PORT was only observed in the cohort of patients treated between 1983 and 1988 but not in the cohort of patients diagnosed from 1989 to 1993. The authors hypothesize that the decrease of cardiac toxicity related to PORT was due to improvements of the thoracic radiotherapy (treatment planning with computed tomography allowing 3 dimensional conformal radiotherapy and high energy delivered by linear accelerator instead of Cobalt).
Overall, radiotherapy modalities used in the randomized trials included in the meta-analysis (13) appear now outdated, especially when compared to the recommendations for planning and delivery of thoracic radiotherapy that have been recently published (50). Latest techniques could further decrease PORT-related toxicity. Image-guided radiotherapy (IGRT) offers several ways to deal with respiratory motion (51,52), such as the deep-inspiration breath-hold radiotherapy (53), the breathing-synchronized radiotherapy or the 4-dimensional CT scan (54) which allows to generate a personalized treatment volume. The clinical results are encouraging (55,56), notably concerning toxicity. Intensity modulated radiotherapy (IMRT) allows better dose distribution compared to conformal radiotherapy (57), with interesting clinical results in inoperable NSCLC (58,59).
Tobacco use seems to be associated with poorer outcome to patients treated by surgery and PORT (60). Gareen et al. (61) have suggested that clinicians have to help patients to quit smoking by giving them specific advice and follow-up instead of a brief injunction.
So, a randomized trial testing PORT for N2 patient was urgently needed…
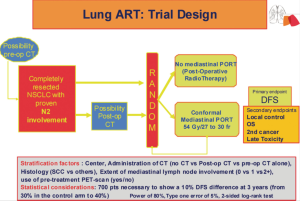
The ongoing phase III Lung Adjuvant Radiotherapy Trial (ART) is randomizing completely resected patients with cytologically or pathologically proven N2 mediastinal disease between PORT and observation (62) (Figure 1). Patients may have had neo-adjuvant chemotherapy, or adjuvant chemotherapy. At initial staging, 18-fluorodeoxyglucose positron emission tomography scanning is recommended (63). In case of pre-operative chemotherapy, the ipsilateral mediastinal involvement has to be pathologically proven before any treatment, so that even in case of mediastinal downstaging (N2 to N1 or N0), the patient can enter the study. Even if induction chemotherapy produces a good response, up to one third of these patients may eventually suffer from local relapse (64), thus it seems interesting to evaluate PORT in this subgroup of patients. There has been a proposal for the definition of complete resection by a group of surgeons of the IASLC (65). Lymph node exploration is mandatory, however the surgeon might choose to use either a simple node sampling or a complete systematic nodal dissection, because the role of these 2 approaches is still debated (66-68). Three-dimensional conformal radiotherapy is, of course, mandatory, together with the use of high-energy photon (6-10 MV) delivered by a linear accelerator. The planned total dose is 54 Gy (37) in fractions of 1.8 or 2 Gy, and the dose per fraction should never exceed 2 Gy (7,38,39). Elective nodal irradiation, which means to treat the whole mediastinum, including the ipsi and contra lateral side of the mediastinum down to the pillars of the diaphragm, and the supra clavicular areas, is not allowed. Treated volume is now limited to involved node station(s) and stations at high risk according to tumor location (40,41,69). This contouring protocol has been evaluated and was able to reduce variability of the treated volume among clinicians (70). Quality assurance procedures, such as a dummy run before any inclusion in a center, aim to verify the compliance to the Lung ART protocol (volume definition, dose to organ at risk, etc.). Indeed, it has been demonstrated that compliance to radiotherapy protocol mustn’t be neglected because it can dramatically impact outcome (71). The main end point of this study is disease free survival (DFS) (24). The 3-year DFS among pN2 patients is about 30%, and the 3-year local recurrence rate is also about 30% (18). In order to observe a 10% absolute improvement of the DFS (from 30% to 40%), the inclusion of 700 patients is planned in the Lung ART study. This study, involving the Intergroupe Francophone de Cancerologie Thoracique (IFCT 0503), has accrued more than 200 patients in France, and has been joined recently by a large national group from the United Kingdom, and the Lung Group, as well as the Radiation Oncology Group from the European Organisation for Research and Treatment of Cancer (EORTC 22055-08053). Another trial is on going in China comparing 4 cycles of adjuvant chemotherapy to 4 cycles of chemotherapy followed by radiotherapy, after complete resection of NSCLC.
In the pre-PET era, the high rate of distant metastases diluted any real effect of local control on overall outcome. As the population of resected N2 patients has changed, because of better selection (more accurate staging with PET CT, brain imaging), better surgery (lung sparing techniques, pre-op and post-op care…), administration of systematic adjuvant or neo-adjuvant chemotherapy which has become standard of care, the major technical advances of radiotherapy may enhance the ability of PORT to improve local relapse free survival and possibly overall survival but this has to be proven. The results of these randomized trials could change the standard care in resected N2 patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol 2008;26:3573-81. [PubMed]
- Kalemkerian GP. Adjuvant therapy for non-small-cell lung cancer. Lancet 2010;375:1230-1. [PubMed]
- Van Houtte P, Rocmans P, Smets P, et al. Postoperative radiation therapy in lung caner: a controlled trial after resection of curative design. Int J Radiat Oncol Biol Phys 1980;6:983-6. [PubMed]
- Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d’Etude et de Traitement des Cancers Bronchiques. Cancer 1999;86:265-73. [PubMed]
- Debevec M, Bitenc M, Vidmar S, et al. Postoperative radiotherapy for radically resected N2 non-small-cell lung cancer (NSCLC): randomised clinical study 1988-1992. Lung Cancer 1996;14:99-107. [PubMed]
- Stephens RJ, Girling DJ, Bleehen NM, et al. The role of post-operative radiotherapy in non-small-cell lung cancer: a multicentre randomised trial in patients with pathologically staged T1-2, N1-2, M0 disease. Medical Research Council Lung Cancer Working Party. Br J Cancer 1996;74:632-9. [PubMed]
- Lafitte JJ, Ribet ME, Prévost BM, et al. Postresection irradiation for T2 N0 M0 non-small cell carcinoma: a prospective, randomized study. Ann Thorac Surg 1996;62:830-4. [PubMed]
- Feng QF, Wang M, Wang LJ, et al. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys 2000;47:925-9. [PubMed]
- Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. The Lung Cancer Study Group. N Engl J Med 1986;315:1377-81. [PubMed]
- Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet 1998;352:257-63. [PubMed]
- Burdett S, Stewart L, PORT Meta-analysis Group. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer 2005;47:81-3. [PubMed]
- Trodella L, Granone P, Valente S, et al. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: definitive results of a phase III randomized trial. Radiother Oncol 2002;62:11-9. [PubMed]
- Mayer R, Smolle-Juettner FM, Szolar D, et al. Postoperative radiotherapy in radically resected non-small cell lung cancer. Chest 1997;112:954-9. [PubMed]
- Phlips P, Rocmans P, Vanderhoeft P, et al. Postoperative radiotherapy after pneumonectomy: impact of modern treatment facilities. Int J Radiat Oncol Biol Phys 1993;27:525-9. [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [PubMed]
- Keller SM, Adak S, Wagner H, et al. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med 2000;343:1217-22. [PubMed]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [PubMed]
- Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol 2013;14:619-26. [PubMed]
- Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 2006;24:2998-3006. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys 2008;72:695-701. [PubMed]
- Scotti V, Meattini I, Saieva C, et al. Post-operative radiotherapy in N2 non-small cell lung cancer: a retrospective analysis of 175 patients. Radiother Oncol 2010;96:84-8. [PubMed]
- Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981-9. [PubMed]
- Burdett SS, Stewart LA, Rydzewska L. Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev 2007;(3):CD006157. [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [PubMed]
- Ichinose Y, Kato H, Koike T, et al. Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer 2001;34:29-36. [PubMed]
- Matsuguma H, Nakahara R, Ishikawa Y, et al. Postoperative radiotherapy for patients with completely resected pathological stage IIIA-N2 non-small cell lung cancer: focusing on an effect of the number of mediastinal lymph node stations involved. Interact Cardiovasc Thorac Surg 2008;7:573-7. [PubMed]
- Urban D, Bar J, Solomon B, et al. Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer. J Thorac Oncol 2013;8:940-6. [PubMed]
- DiBiase SJ, Werner-Wasik M, Croce R, et al. Standard off-cord lung oblique fields do not include the entire mediastinum: a computed tomography simulator study. Am J Clin Oncol 2000;23:249-52. [PubMed]
- Machtay M, Lee JH, Shrager JB, et al. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clin Oncol 2001;19:3912-7. [PubMed]
- Cosset JM, Henry-Amar M, Girinski T, et al. Late toxicity of radiotherapy in Hodgkin’s disease. The role of fraction size. Acta Oncol 1988;27:123-9. [PubMed]
- Dubray B, Henry-Amar M, Meerwaldt JH, et al. Radiation-induced lung damage after thoracic irradiation for Hodgkin’s disease: the role of fractionation. Radiother Oncol 1995;36:211-7. [PubMed]
- Watanabe Y, Shimizu J, Tsubota M, et al. Mediastinal spread of metastatic lymph nodes in bronchogenic carcinoma. Mediastinal nodal metastases in lung cancer. Chest 1990;97:1059-65. [PubMed]
- Kelsey CR, Light KL, Marks LB. Patterns of failure after resection of non-small-cell lung cancer: implications for postoperative radiation therapy volumes. Int J Radiat Oncol Biol Phys 2006;65:1097-105. [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [PubMed]
- Chapet O, Kong FM, Quint LE, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys 2005;63:170-8. [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949-55. [PubMed]
- Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956-62. [PubMed]
- Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005;97:419-24. [PubMed]
- Wakelee HA, Stephenson P, Keller SM, et al. Post-operative radiotherapy (PORT) or chemoradiotherapy (CPORT) following resection of stages II and IIIA non-small cell lung cancer (NSCLC) does not increase the expected risk of death from intercurrent disease (DID) in Eastern Cooperative Oncology Group (ECOG) trial E3590. Lung Cancer 2005;48:389-97. [PubMed]
- Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2007;110:911-7. [PubMed]
- De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 2010;28:5301-10. [PubMed]
- Verellen D, De Ridder M, Linthout N, et al. Innovations in image-guided radiotherapy. Nat Rev Cancer 2007;7:949-60. [PubMed]
- Paumier A, Crespeau A, Krhili S, et al. Dosimetric study of the different techniques to deal with respiratory motion for lung stereotactic radiotherapy. Cancer Radiother 2012;16:263-71. [PubMed]
- Hanley J, Debois MM, Mah D, et al. Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys 1999;45:603-11. [PubMed]
- Hof H, Rhein B, Haering P, et al. 4D-CT-based target volume definition in stereotactic radiotherapy of lung tumours: comparison with a conventional technique using individual margins. Radiother Oncol 2009;93:419-23. [PubMed]
- Giraud P, Djadi-Prat J, Morvan E, et al. Dosimetric and clinical benefits of respiratory-gated radiotherapy for lung and breast cancers: results of the STIC 2003. Cancer Radiother 2012;16:272-81. [PubMed]
- Giraud P, Morvan E, Claude L, et al. Respiratory gating techniques for optimization of lung cancer radiotherapy. J Thorac Oncol 2011;6:2058-68. [PubMed]
- Rousseau D, Autret D, Krhili S, et al. Are there any dosimetric advantages in using VMAT for treatment of locally advanced non-small cell lung cancer? Cancer Radiother 2012;16:619-26. [PubMed]
- Scorsetti M, Navarria P, Mancosu P, et al. Large volume unresectable locally advanced non-small cell lung cancer: acute toxicity and initial outcome results with rapid arc. Radiat Oncol 2010;5:94. [PubMed]
- Liao ZX, Komaki RR, Thames HD Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010;76:775-81. [PubMed]
- Nguyen SK, Masson-Côté L, Fortin A, et al. Influence of smoking status on treatment outcomes after post-operative radiation therapy for non-small-cell lung cancer. Radiother Oncol 2010;96:89-93. [PubMed]
- Gareen IF, Park ER, Gorelick J, et al. The effects of physician-delivered brief smoking cessation on ACRIN/NLST participants’ smoking behaviors. J Clin Oncol 2013;13:abstr 7525.
- Le Péchoux C, Dunant A, Pignon JP, et al. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. J Clin Oncol 2007;25:e10-1. [PubMed]
- Ung YC, Maziak DE, Vanderveen JA, et al. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 2007;99:1753-67. [PubMed]
- Amini A, Lou F, Correa AM, et al. Predictors for locoregional recurrence for clinical stage III-N2 non-small cell lung cancer with nodal downstaging after induction chemotherapy and surgery. Ann Surg Oncol 2013;20:1934-40. [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [PubMed]
- Yl Wu. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [PubMed]
- Wright G, Manser RL, Byrnes G, et al. Surgery for non-small cell lung cancer: systematic review and meta-analysis of randomised controlled trials. Thorax 2006;61:597-603. [PubMed]
- Kiricuta IC, Mueller G, Stiess J, et al. The lymphatic pathways of non-small cell lung cancer and their implication in curative irradiation treatment. Lung Cancer 1994;11:71-82. [PubMed]
- Spoelstra FO, Senan S, Le Péchoux C, et al. Variations in target volume definition for postoperative radiotherapy in stage III non-small-cell lung cancer: analysis of an international contouring study. Int J Radiat Oncol Biol Phys 2010;76:1106-13. [PubMed]
- Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol 2010;28:2996-3001. [PubMed]