The prognostic value of metformin for advanced non-small cell lung cancer: a systematic review and meta-analysis
Introduction
Lung cancer is the leading cause of cancer morbidity and mortality in males, and also the second leading cause of mortality in females worldwide (1). There are two cancer types, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), accounting for 84% and 16% of all lung cancer cases, respectively (2). The 5-year survival rate of stage IA NSCLC is 73%, but the 5-year survival rate of stage IV decreases to 13% (3).
Diabetes mellitus (DM) is another common disease worldwide. According to the current report of World Health Organization, there were approximately 422 million cases of diabetes in 2014, its global prevalence had risen from 4.7% in 1980 to 8.5% in 2014 among adults over 18 years of age; it is projected to be the seventh leading cause of mortality in 2030 (3). DM is a major cause of other diseases, including kidney failure, diabetes-related heart diseases, lower limb amputation, etc., threatening life expectancy and quality of life.
To successfully cure early-stage NSCLC and even prolong survival for late-stage NSCLC patients, in current stage a number of therapeutic strategies have been developed and implemented, such as surgery, chemotherapies, targeted therapies and immunotherapies. Nevertheless, the condition becomes complex for treatments when patients have other diseases like diabetes concurrently with lung cancer.
Normally, Metformin is the first-line treatment for uncomplicated cases of type 2 diabetes. For patients with diabetes, three meta-analyses (one based on systematic review) have demonstrated significantly lower risk of cancer with Metformin compared to non-Metformin use (4-6). Accordingly, a hypothesis that Metformin may have anti-cancer effects on lung cancer was proposed. It is worthy of acknowledgement that, Metformin has been proved as a safety profile for patients. More importantly, compared with other anti-cancer treatments for lung cancer, its price is affordable. Therefore, even though the mechanism between Metformin and lung cancer is not completely understood yet, further studies have been implemented to investigate whether Metformin could prolong the survival of lung cancer patients.
Based on three current meta-analyses (one based on systematic review), potential efficacy of Metformin to prolong overall survival (OS) could be found in SCLC consistently. The pooled hazard ratio (HR) was 0.52 (P<0.05) in the three studies (7-9). However, for NSCLC their outcomes were controversial. In the systematic review and meta-analysis, Metformin significantly improved both OS and progression-free survival (PFS) of diabetic NSCLC patients (7). The pooled HR was 0.77 (0.71–0.84) (P<0.01) and 0.53 (0.39–0.71) (P<0.01), respectively. A meta-analysis presented similar findings [OS: HR =0.75 (0.58–0.97) P=0.03] (8). However, an inconsistent outcome was found in another meta-analysis, in which Metformin did not improve OS of NSCLC + DM patients [HR =1.06 (0.51–2.19), P=0.88] (9).
From these studies, we also recognized that patients with different cancer stages were eligible for meta-analyses. In this condition, the patients’ survival may not directly reflect the real efficacy between different treatment strategies for cancer. Because, other causes of mortality may exist in addition to lung cancer itself (such as different complications of diabetes), especially in early-stage NSCLC, these other causes may contribute further effects to patients’ life quality and survival due to longer life expectancy. Namely, if above meta-analyses only investigated the Metformin and non-Metformin usage in patients who had advanced or inoperable NSCLC patients (instead of early-stage NSCLC), with a shorter life expectancy, the survival of both groups would be more reflected by the Metformin usage, instead of other causes mentioned above. Considering the complexity of the patients with both NSCLC and DM, we assume one of the ideal strategies is to comparing Metformin usage and non-Metformin usage in NSCLC patients who do not have diabetes. In this way, the effects from DM could be attenuated.
Given the above consideration based on current meta-analyses, in this systematic review and meta-analysis, we aim to investigate the efficacy of Metformin on advanced or inoperable NSCLC patients, including the patients with or not with diabetes in our eligible studies.
Methods
Search strategy and study selection
Based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (10), articles were searched and selected by JRZ. Databases included PubMed, Scopus and Web of Science, and the time interval was from the inception date of the databases to 1 September 2017. The search formula was: (((Lung Cancer[Title/Abstract]) AND Metformin[Title/Abstract])) AND (((((Advanced) OR Metastatic) OR Inoperable) OR Unresectable) OR Stage IV) [English] [Journal Article].
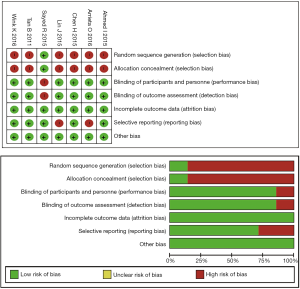
In our research, the inclusion criteria for eligible studies is: (I) the studies should be a cohort study or randomized controlled trial; (II) the studies should focus on advanced or inoperable NSCLC patients, instead of early-stage NSCLC or SCLC; (III) the experimental group should involve Metformin usage, and the control group should not have Metformin usage; (IV) the studies should have the survival data based on the comparison of Metformin usage and non-Metformin usage, including PFS and OS. The articles of these studies should be a full-length research paper published in an academic journal; the articles written in other languages instead of English were excluded. The quality of our included articles was assessed according to the Cochrane Collaboration’s tool (Figure S1). Endnote X7 (Thomson Reuters, New York, US) was used during this process.
Data extraction
The following characteristics of each trial were extracted: author, published year, study design, anti-cancer treatments, cancer stage, comparison with basic population (with or without diabetes), patient number, median PFS and corresponding HR, and median OS with its HR. For these, JRZ firstly extracted, and JYW checked the data.
Statistical analysis
Data synthesis was conducted based on the Generic Inverse Variance method and the fixed-effect model in Review Manager 5.3 (The Cochrane Collaboration, London, England). In this process, if the corresponding p value for heterogeneity was less than 0.05 or the I2 index (I2) was over 50%, we used a random-effect model instead of the fixed-effect model, to reduce the heterogeneity effect on data synthesis. The pooled HR was the outcome: HR less than 1 indicated patients with Metformin usage had longer PFS or OS than patients without Metformin usage. Significant difference was indicated if P value was less than 0.05. Subgroup analysis was conducted based on cancer stage (local advanced versus advanced).
Results
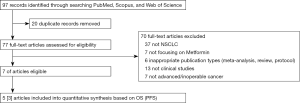
From a total of 97 articles in electronic databases, we included seven eligible articles (Figure 1) (11-17). Among them, only one was a randomized controlled trial, and the rest were retrospectively cohort studies based on existing databases. Basic characteristics of all included studies were summarized in Table 1.

Full table
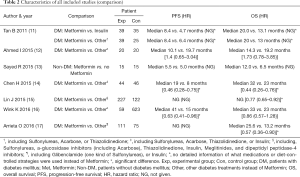
Characteristics of comparison data in included studies is demonstrated in Table 2. There was only one study comparing Metformin usage and non-Metformin usage in NSCLC patients without DM. There was no significant difference on either OS or PFS (13). The remaining studies focused on the comparison of Metformin usage and non-Metformin usage in patients with concurrent NSCLC and DM, including one study that specifically compared Metformin with insulin or other DM treatments (instead of Metformin and Insulin) (11). Among them, a significant difference in OS and PFS could be found in four and three studies, respectively.
Full table
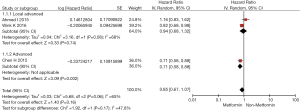
Results of data synthesis based on patients with both NSCLC and DM are presented in Figures 2 and 3. Overall, patients with Metformin usage had longer OS and PFS than patients without Metformin usage, but a significant difference could be only found in OS [OS: pooled HR =0.87 (0.77–0.99), P=0.04; PFS: HR =0.85 (0.67–1.07), P=0.16]. In subgroup analysis, a significant prolonged survival was shown among advanced cancer patients with Metformin usage compared with non-Metformin usage, regardless of OS [pooled HR =0.81 (0.70–0.94), P<0.01) or PFS (pooled HR =0.71 (0.58–0.88), P<0.01]; however, among local advanced cancer patients, no significantly longer OS and PFS was found in Metformin usage group [OS: pooled HR =1.05 (0.79–1.40), P=0.74; PFS: HR =0.94 (0.68–1.32), P=0.74].
Discussion
As we acknowledge, this systematic review and meta-analysis is the first to investigate the value of Metformin for advanced or inoperable NSCLC patients. We found that, the NSCLC and DM patients with Metformin usage had significantly longer OS than the NSCLC and DM patients without Metformin usage. In addition to this comparison, both significantly longer PFS and OS could be found in diabetic patients with advanced NSCLC, instead of local advanced NSCLC.
Even though sufficient understanding of the association between Metformin and lung cancer has not been completely confirmed, some known mechanisms may account for the above findings. (I) Metformin activates the adenosine mono-phosphate activated protein kinase (AMPK) pathway. AMPK is a highly-conserved serine/threonine protein kinase that plays an important role in protecting cellular functions under energy restricted conditions (18). Sanchez-Cespedes and his team found that about 30% of NSCLC contain the mutant liver kinase B1 (LKB1) gene (19), which is an upstream signal of AMPK. The mutant LKB1 will lead to AMPK dysfunction. Studies showed that Metformin actives AMPK by specifically inhibiting mitochondrial respiratory complex I (complex I) (20,21). This inhibition leads to a decline of adenosine triphosphate (ATP) in cells directly and deteriorates the balance of ATP production and consumption. As a result, AMPK is activated by increasing the intercellular AMP to ATP ratio (18). AMPK activation leads to essential metabolic enzymes phosphorylation and transcription factors or co-activators modulating gene expression, which inhibits glucose, fat and protein synthesis in tumor cells. Thus, the AMPK pathway could inhibit tumor cell proliferation by regulating inflammatory response in the tumor microenvironment (22), especially in inhibiting the expression of cyclooxygenase-2 (COX-2) (23). This finding demonstrates Metformin has potential anticancer ability in the tumor microenvironment. (II) Metformin inhibits insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF-1R) in NSCLC. The IR and IGF-1R pathway activates cell proliferation and mitosis by activating phosphatidyl inositol 3-kinase (PI3K) and protein kinases B (Akt). Memmott RM et al., have found that Metformin decreased phosphorylation of IGF-1 and/or IR in lung tissue and in circulation via mTOR pathways, instead of AMPK (24). This AMPK-independent pathway could also eliminate downstream signaling and inhibit cancer cell proliferation (23).
From this study, cautious interpretation is needed. First, our meta-analysis was conducted based the comparison of Metformin usage and non-Metformin usage in patients with NSCLC and DM. As we acknowledge, Metformin is the first-line treatment for type 2 DM, especially in the early stage. In other words, a mono strategy is not efficacious when diabetes deteriorates to the late stage. In this condition, other treatments need to be considered, like Insulin. Therefore, we assume that, perhaps the patients’ disease burden in the non-Metformin group may be higher than the burden in the Metformin group, to some extent leading to inferior survival in the non-Metformin group. With this assumption, according to our study and two previous meta-analyses with positive results (7,8), it is still difficult to conclude that Metformin presents an anti-cancer effect on patients with NSCLC and DM, and prolongs their survival.
To more exactly investigate the value of Metformin for NSCLC, one of the ideal strategies is to compare Metformin usage and non-Metformin usage in NSCLC patients who do not have diabetes. In this situation, the stage of diabetes and the effects of other diabetic treatments could unnecessarily impact the final treatment effect. As of now, we only recognize one study based on this population for comparison (13). The result showed that patients using Metformin had longer PFS and OS compared with patients not using Metformin, even though no statistically significant difference was indicated. We consider the sample size was too small (both 15 patients in experiential and control arms), in order to attenuate the statistical power of treatment effect. However, this study still indicates the potential value of Metformin for treating NSCLC, which warrants future larger studies to confirm.
Second, the interaction between Metformin and other anti-cancer treatments on diabetic NSCLC patients is unknown in our study. Interestingly, our included studies presented different treatment effects of OS between Metformin usage and non-Metformin usage in patients with both NSCLC and DM. In one study, patients in both groups received epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) to treat cancer, and the treatment effect between Metformin usage and non-Metformin usage presented the most superiority in favor of Metformin usage among all included studies (14). The second superiority of the treatment effect was in the study whose patients used chemotherapy or TKI (17). The third superiority was in the study using chemotherapy only (15), and the worst were in the two studies based on chemoradiotherapy. Considering our findings and some assumptions written in this paper, we are pleased to acknowledge a multicenter, double-blind, randomized controlled trial (NCT01864681) (25). This trial investigates the value of Metformin combined with Gefitinib (one of EGFR TKIs) as the first-line treatment for stage IIIb–IV, EGFR-mutated NSCLC patients. Also, in this trial, patients who have diabetes would be excluded. Based on the statistical design, a larger sample size (nearly 200 patients) would be enrolled.
There are some limitations in our research. Even though we have already narrowed our scope on advanced or inoperable NSCLC instead of involving SCLC and early-stage NSCLC, the different baseline settings among our included studies could limit the generalization of our research, such as adjusted factors for their statistical analyses, treatments for diabetes in non-Metformin groups, as well as other factors we have mentioned, like diabetes stage, NSCLC histopathological subtypes and different treatments for cancer. Moreover, we don’t know whether there were other treatments used in the experimental and control groups of all included studies, because both advanced (or inoperable) NSCLC and DM could be complex diseases with different complications. The mentioned limitations above would be more evident in a retrospective cohort study. Given that all of our included studies for meta-analysis are based on the retrospective cohort study design, therefore the robustness of our meta-analysis is inevitable affected by these limitations.
Furthermore, in our data synthesis, the treatment effect in four of five studies was in favor of Metformin usage (except one whose treatment effect was in favor of non-Metformin usage), but the effect of heterogeneity in the data synthesis of our study was not low: the index (I2) was over 50% in both overall and subgroup syntheses. This attenuates the confidence of our result. Last, the number of the studies we included is small (7 included, and only 5 eligible for meta-analysis), even though we have extended our searching from PubMed to other large databases (Scopus, Web of Science). This situation also limits us to evaluate potential confounders through subgroup analysis in this study-level research, in order to analyze the causes of the heterogeneity in our data synthesis.
In conclusion, NSCLC and DM patients with Metformin usage had longer survival than the patients without Metformin usage. This superiority was more significant in the advanced stage instead of the local advanced stage. However, considering different factors impacting the value of Metformin for advanced NSCLC, further investigation is needed.
Acknowledgements
We thank Ms. Carolyn Smith (Senior Tutor, The Writing Center, Washington University in St. Louis), Ms. Elizabeth Burke (Instructor, Office for International Students and Scholars, Washington University in St. Louis), Dr. Amy Eyler (Associate Professor, George Warren Brown School, Washington University in St. Louis), and Ms. Anjana Delhi (M.P.H. Candidate, George Warren Brown School, Washington University in St. Louis) for providing suggestions on manuscript revision. We also sincerely thank all authors, investigators, sponsors and patients for their effort and participation in our included studies.
Funding: This study is supported by the Civil Science Major Foundation of Science and Information Bureau of Guangzhou (No. 2011Y2-00024).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [Crossref] [PubMed]
- Zhang ZJ, Bi Y, Li S, et al. Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. Am J Epidemiol 2014;180:11-4. [Crossref] [PubMed]
- Zhu N, Zhang Y, Gong YI, et al. Metformin and lung cancer risk of patients with type 2 diabetes mellitus: A meta-analysis. Biomed Rep 2015;3:235-41. [Crossref] [PubMed]
- Wang L, Song Y, Wu GN, et al. Association of the metformin with the risk of lung cancer: a meta-analysis. Transl Lung Cancer Res 2013;2:259-63. [PubMed]
- Cao X, Wen ZS, Wang XD, et al. The Clinical Effect of Metformin on the Survival of Lung Cancer Patients with Diabetes: A Comprehensive Systematic Review and Meta-analysis of Retrospective Studies. J Cancer 2017;8:2532-41. [Crossref] [PubMed]
- Wan G, Yu X, Chen P, et al. Metformin therapy associated with survival benefit in lung cancer patients with diabetes. Oncotarget 2016;7:35437-45. [Crossref] [PubMed]
- Tian RH, Zhang YG, Wu Z, et al. Effects of metformin on survival outcomes of lung cancer patients with type 2 diabetes mellitus: a meta-analysis. Clin Transl Oncol 2016;18:641-9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Tan BX, Yao WX, Ge J, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer 2011;117:5103-11. [Crossref] [PubMed]
- Ahmed I, Ferro A, Cohler A, et al. Impact of metformin use on survival in locally-advanced, inoperable non-small cell lung cancer treated with definitive chemoradiation. J Thorac Dis 2015;7:346-55. [PubMed]
- Sayed R, Saad AS, El Wakeel L, et al. Metformin Addition to Chemotherapy in Stage IV Non-Small Cell Lung Cancer: an Open Label Randomized Controlled Study. Asian Pac J Cancer Prev 2015;16:6621-6. [Crossref] [PubMed]
- Chen H, Yao W, Chu Q, et al. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett 2015;369:97-102. [Crossref] [PubMed]
- Lin JJ, Gallagher EJ, Sigel K, et al. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med 2015;191:448-54. [Crossref] [PubMed]
- Wink KC, Belderbos JS, Dieleman EM, et al. Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother Oncol 2016;118:453-9. [Crossref] [PubMed]
- Arrieta O, Varela-Santoyo E, Soto-Perez-de-Celis E, et al. Metformin use and its effect on survival in diabetic patients with advanced non-small cell lung cancer. BMC Cancer 2016;16:633. [Crossref] [PubMed]
- Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253-70. [Crossref] [PubMed]
- Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res 2002;62:3659-62. [PubMed]
- Zhang Y, Storr SJ, Johnson K, et al. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget 2014;5:12936-49. [PubMed]
- Leclerc GM, Leclerc GJ, Kuznetsov JN, et al. Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in ALL lymphoblasts. PLoS One 2013;8:e74420. [Crossref] [PubMed]
- Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta 2000;1470:M69-78. [PubMed]
- Lee JY, Choi AY, Oh YT, et al. AMP-activated protein kinase mediates T cell activation-induced expression of FasL and COX-2 via protein kinase C theta-dependent pathway in human Jurkat T leukemia cells. Cell Signal 2012;24:1195-207. [Crossref] [PubMed]
- Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066-76. [Crossref] [PubMed]
- Li KL, Li L, Zhang P, et al. A Multicenter Double-blind Phase II Study of Metformin With Gefitinib as First-line Therapy of Locally Advanced Non-Small-cell Lung Cancer. Clin Lung Cancer 2017;18:340-3. [Crossref] [PubMed]