Breath analysis as a diagnostic and screening tool for malignant pleural mesothelioma: a systematic review
Introduction
Asbestos
Asbestos fibres are fibrous silicate minerals, widely used in construction during the 20th century due to its strong fire, chemical and abrasion resistance (1,2). Asbestos is a collective name for different fibre groups characterized by specific features: a serpentine (chrysotile) and 5 amphiboles (crocidolite, amosite, anthophyllite, actinolite and tremolite) (1,2). Asbestos fibres cause cancer of the lung, larynx, ovaries and mesothelium (mesothelioma), as well as benign disease like asbestosis, pleural plaques and thickening and effusion in the pleura (3). Approximately 95% of all globally used asbestos products are chrysotile. Nevertheless, although chrysotile fibres are thought to be less carcinogenic, all asbestos fibres have been shown to be carcinogenic and are classified a group 1 human carcinogen by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) (1,2,4).
Asbestos-related diseases (ARD)
Asbestos can cause benign ARD, like asbestosis and pleural plaques. Asbestosis is identified as bilateral disseminated interstitial fibrosis of the lungs, but not of the pleura (5). The presence of this disease indicates a heavy previous exposure to asbestos fibres. Unlike for mesothelioma, there is a safe exposure threshold of 25–100 fibres/mL/year, under which asbestosis does not occur (6). The median latency period is 31 years, depending on the intensity of the exposure. The more intense the exposure, the more likely asbestosis will occur (7,8). Pleural plaques are the most common manifestation of asbestos exposure (9). Plaques are small areas of hyaline fibrosis which can become calcified and mostly originate on the parietal pleura of the chest wall and diaphragm (10). Because of the layers of hyalinised collagen fibres, plaques appear white and are often multiple and bilateral (11,12). Plaques occur with lower inhaled asbestos burdens and can result from small temporally exposures. Plaques appear 20–30 years after exposure in 50–60% of individuals with a heavy or prolonged asbestos exposure and serve as a marker of asbestos exposure (9,11).
Malignant pleural mesothelioma (MPM) is a rare, aggressive and treatment-resistant tumour originating from the serosal cells lining the lungs (13). MPM has a median latency time of 40–50 years between first exposure to asbestos fibres and its diagnosis (14). There is a proven link between an increased exposure to asbestos and the development of MPM (15). However, there is no safe threshold where under MPM will not develop. A single, intensive exposure is therefore enough to induce MPM (5).
The incidence rates of MPM worldwide are highest in the United Kingdom, Australia, The Netherlands, New Zealand, Malta and Belgium, where the incidence ratio is at least 2 per 100,000 capita (16). In 2015, 301 people have been diagnosed with MPM in Belgium of which 238 were male (17,18). According to the WHO, mesothelioma, asbestos-related lung cancer and asbestosis globally cause 107,000 deaths annually. In 2005, occupational asbestos exposure was estimated to cause 43,000 deaths worldwide due to MPM and 7,000 deaths due to asbestosis (19). Given to the long interval between the first causative asbestos exposure and the diagnosis, the incidence rate of MPM is expected to rise in the next decades (20).
Patients experience non-specific symptoms, like dyspnoea and thoracic pain (21), that contribute to delaying diagnosis to advanced stage disease in mostly elderly persons with a median age at diagnosis of 65 years (22). MPM has a very high mortality rate (12). After diagnosis is made, untreated patients have a median survival of about 9 months which can be increased to 12 months with standard-of-care chemotherapy (23). This is an alarming fact and it is assumed that an early diagnosis could possibly contribute to improve this low survival rate, as seen for lung cancer (24).
There is no curative treatment and treatment is limited to palliative chemotherapy. Patients with a good performance benefit from this treatment with an increased survival of around two months and an improvement of symptoms like pain and shortness of breath (25). The standard-of-care chemotherapy consists of the combination of an antifolate (pemetrexed) and a platinum-derivate (cisplatin) (26). However, the addition of bevacizumab (angiogenesis inhibitor) to the standard-of-care recently showed to increase the median overall survival of patients to 18 months (27). The upcoming field of immunotherapy as experimental treatment for mesothelioma furthermore holds promise for future improvement on survival by MPM treatment (28,29). The added value of these new treatment options should be further investigated in order to become the new standard-of-care (26,27,30,31).
Pathophysiology
Inhaling asbestos fibres causes chronic inflammation and oxidative stress. First, chronic inflammation is caused by the extended phagocytic activity of macrophages engulfing asbestos fibres, called frustrated phagocytosis. This induces fibrosis, thereby generating reactive oxygen species (ROS), resulting in asbestosis (8). However, this fibrosis can also be malignant. Therefore, people suffering from asbestosis have an increased risk for developing MPM and lung cancer (32). Second, the asbestos fibres have a high iron content that triggers Fenton-like chemical reactions, leading to constantly maintaining the induction of ROS (33), leading to cellular and DNA damage. MPM is usually classified into three subtypes according to histology: biphasic, sarcomatoid and epithelioid (10). Epithelioid MPM is the most common subtype and these patients have a better overall survival in comparison with patients who suffer from other MPM subtypes (34).
Diagnosis of asbestos-related diseases
The diagnosis of asbestosis is mainly made non-invasively based upon clinical and radiological grounds. It is therefore mandatory to document previous asbestos exposure whereafter a physical examination can determine interstitial fibrosis by hearing crackles on auscultation of the lungs. Further diffuse opacities are detectable on radiologic examination. At last, a lung function test will indicate impairment. Analysis of a biopsy is rarely required (35).
Currently, imaging tests can suggest the presence of MPM after a thorough anamneses. Usually, a unilateral pleural effusion is seen on chest X-ray. The sensitivity for detecting MPM with a chest X-ray depends on the location, shape, size and calcification of the tumour and the technical quality of the X-ray. For this reason, it is less sensitive for detecting pleural diseases (especially pleural plaques) than a chest CT-scan, which is the gold standard for the evaluation and the follow-up of MPM (11). However, only a histopathological examination of a biopsy obtained after an invasive thoracoscopy allows to make a definitive MPM diagnosis (33). Consequently, non-invasive biomarkers that may contribute to an earlier diagnosis are of great interest.
Blood biomarkers
Blood has been studied to look for biomarkers for MPM and has been reviewed previously (33). One of the most studied biomarkers is serum mesothelin-related protein (SMRP). Mesothelin is a surface protein which plays a role in mesothelial cell adhesion and becomes the circulating blood product SMRP after cleavage. In 60% of MPM patients, an elevation of SMRP was seen (25,36). However, despite a high specificity, SMRP lacks sensitivity. Therefore, further research focused on the combination of SMRP with other biomarkers in order to improve the diagnostic accuracy for early diagnosis of MPM (37). Moreover, the histology is an important determinant of prognosis of MPM. Some studies found an inverse relation between SMRP and overall survival, but the prognostic impact on overall survival was lost when limited to epithelial MPM (38). Furthermore, MPM patients with recurrence or progression after initial treatment had the highest values of SMRP. As a result, SMRP concentrations could be used as a tool for monitoring patient’s response to treatment (39).
Other research focused on serum and plasma osteopontin (OPN) as second important biomarker (40). SMRP and OPN are both promising biomarkers for diagnosis of MPM (41), but the specific diagnostic accuracy remains insufficient, restricting the use of these compounds in clinical practice (40,42). Other blood biomarkers are also potentially useful: fibulin-3, high mobility group box 1 (HMGB1), aptamers [SOMAmer (Slow Off-rate Modified Aptamer)] and micro-RNA’s (38). In some studies, higher plasma fibulin-3 levels were found in MPM patients than in control groups (43). Hyperacetylated HMGB1 could have a role as a potential diagnostic marker to differentiate MPM patients form asbestos-exposed individuals and healthy controls (HC) (38,44). Finally, using SOMAmers as capture agents, MPM was detected in a group of asbestos-exposed persons with 92% accuracy, and its sensitivity was found associated with pathological stage (38). These results provide a probable use for early diagnosis of MPM in a high risk population. Although some of the abovementioned biomarkers show diagnostic potential, none of these are validated and therefore are not currently implemented in clinical practice to screen potential individuals at risk for MPM. This urges the need to continue the search for accurate biomarker to enable an early stage diagnosis of MPM. Therefore, the field of breath analysis can be explored.
Exhaled breath
Composition
Exhaled breath contains a liquid phase (water vapor) and a gaseous phase including nitrogen, oxygen, carbon dioxide, inert gases, and a small function of volatile organic compounds (VOCs) (45). VOCs are lipophilic components with low molecular weight (<300 Da) and have a high vapor pressure at a temperature of approximately 20 °C (46). They are released from the human body and can be detected through urine, skin, blood and exhaled breath (47). In a typical population, a single breath sample contains around 200 different VOCs, mostly at picomolar range. In total, more than 3,000 different VOCs are already described (45). Besides organic compounds, breath can contain inorganic compounds, such as NO (48).
The liquid phase encloses exhaled breath condensate (EBC) and aerosols (49), containing different diluted non-volatile molecules ranging from simple ions to DNA, leukotrienes, C-reactive protein, lipid, microbiota, etc. Hence, for analyzing these biomarkers, EBC is the best approach (50).
Origin of VOCs
VOCs can originate from exogenous sources via inhalation and skin adsorption, but, more importantly, can also arise from the physiological and pathophysiological processes in the body, such as inflammation, oxidative stress and fat metabolism (51). Endogenously formed VOCs enter the bloodstream and are transported to the lungs. Through the gas exchange mechanisms in the lung alveoli, VOCs are released in the breath (52). Hence, the composition and concentration of breath VOCs can be used as easy, non-invasive biomarkers that reflect the metabolic changes and pathologic processes throughout the body.
VOCs as biomarkers for disease
As with blood biomarkers, a single breath compound is expected to be insufficient to obtain enough information about environmental exposure or chronic diseases, due to the complex underlying pathophysiological processes of disease. This urges to explore the total amount of exhaled VOCs, thereby focussing on biomarker panels, and allowing a subsequent identification and enrichment of VOCs of interest. In that way, a unique VOC pattern can be generated, which has shown to be promising to detect infectious diseases (pneumonia, H. pylori infection), cancer (prostate cancer, colorectal cancer,…), pulmonary diseases (asthma, chronic obstructive pulmonary disease), cardiovascular diseases, gastro-intestinal diseases (gastric carcinoma, gastric ulcer, gastritis) and liver diseases (53-61). This is of particular interest because the abovementioned diseases relate to various metabolic processes in the body. Since patients can have comorbidities, collecting the plethora of VOCs can allow to find information about comorbidities next to the disease of interest (47).
The pathogenesis of a human tumour is a multistep process in which cancer cells acquire several biological characteristics, called hallmarks of cancer, that allow a normal cell to become malignant. These include proliferative signalling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis and activating invasion and metastasis (62). Inflammatory cells foster these hallmarks and therefore promote tumour development (62). This changes cell metabolism and, hence, a change in VOC production is to be expected. Furthermore, oxidative stress is a vital risk factor for the development of cancer (47). Since inflammation is a hallmark of cancer, excessive ROS production occurs due to lipid peroxidation and induction of cytochrome P450 enzymes, and thereby influencing VOCs. Above of this, many metabolic pathways change the VOC production in the body because they are (over)activated in the occurrence of cancer. Hydrocarbons (alkanes, alkenes), alcohols, aldehydes, ketones, esters, nitriles and aromatic compounds are found to be cancer-related VOCs, but they can also be related to other inflammatory processes or be induced by other sources, such as tobacco smoking (47), thereby limiting their use as stand-alone markers in favour of more informative biomarker panels.
Since asbestos fibres induce chronic inflammation and oxidative stress that leads to MPM, this will result in raising the cell’s metabolic activity and the production of ROS. This will ultimately lead to a change in the VOC production and proposes that VOCs can be used as non-invasive diagnostic biomarkers for disease. Given the ease and non-invasiveness of sampling, this systematic review will therefore survey the existing knowledge of how to use volatile biomarkers in breath for the management of MPM and asbestosis.
Breath analysis: methods
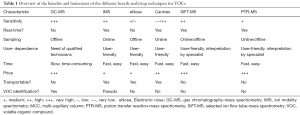
Because the current diagnostic work-up of MPM and many other cancers suffers from limitations like long latency or invasiveness, there is need for a new test that is fast and non-invasive. Hence, in contrast to current diagnostic methods, breath analyzing techniques are promising where only a breath sample is needed. Given this non-invasive and practical sampling, research has recently led to the rise of a high-throughput breathomics era, using different techniques to analyse VOCs in breath, all having their benefits and limitations (Table 1).
Full table
The first technique, gas chromatography-mass spectrometry (GC-MS), is the gold standard in breath analysis. This offline method makes it possible to separate, quantify and identify the individual compounds of a gaseous mix (46). The breath is collected into bags or onto fibres [solid-phase micro-extraction (SPME) fibres] or thermal desorption tubes where VOCs are concentrated (33). The VOCs are desorbed and separated over a heated GC-column based upon their chemical characteristics (51). Afterwards, the VOCs are ionised and fragmented in the MS, allowing to identify and quantify the VOCs. Despite the fact that GC-MS allows quantitative analysis, it is time-consuming, immobile, costly and there is a need of an trained expert operator (33).
Next to this, there are handheld electronic noses (eNoses). This sensor technology allows to recognize the bulk of VOCs as a breath pattern or ‘smellprint’ and is based upon human olfactory perception. Hence, eNoses allow bedside sampling and fast analysis but do not allow specific VOC identification.
Another technique to analyse VOCs is ion mobility spectrometry (IMS). With this online method, the patient breathes directly into the IMS device. The velocity of the VOCs to traverse a drift tube under influence of an electrical field and a counter gas is measured, and will relate to the VOCs’ size, charge, shape and mass (the so-called ion mobility). This allows the separation of compounds (33) and individual VOC identification becomes possible due to the coupling with a multi-capillary column (MCC) (51). This technique combines the advantages of GC-MS and eNose sampling by allowing a fast and low cost analysis, sampling at the patients’ bedside and the identification of compounds.
Canine scent, is based on the capacity of dogs to smell and discriminate between different breath samples. It is relatively expensive and there is no VOC quantification or identification possible (51). Furthermore, selected ion flow tube-mass spectrometry (SIFT-MS), allows one to quantify a range of VOCs in air and breath in a single test. This technique is based on the principle of chemical ionization (63) and potentially allows for a fast assessment of occupational exposure in real time (64,65). The main limitation is the uncertain quantification and identification of analyte ions, and the fact that it is expensive and space-consuming (63,65). Lastly, proton transfer reaction-mass spectrometry (PTR-MS) is also based on the principal of chemical ionization whereby analytes are characterized according to mass/charge ratio. It is accurate, quick, less time consuming, ideal for complex gas mixtures and can be used as an online breath analyzing technique (63). The downside is that identification of compounds is not possible, and that it is also space-consuming and expensive (63).
Goals of the systematic review
Because the current methods for diagnosing MPM hamper an efficient diagnosis and curative treatment (late diagnosis, expensive and/or invasive procedures), there is a strong need for a non-invasive tool which may allow earlier diagnosis. Hence, given that blood biomarkers have not proven to be useful for early detection of surviving, and breath analysis is a new, innovative field of research, we want to explore the current knowledge of using breath analysis for the management and diagnosis of MPM and asbestosis. Our findings are summarized in this systematic review according to the recommendations of the Cochrane Collaboration for diagnostic research.
Materials and methods
Search strategy
We searched for studies concerning subjects with MPM or asbestosis compared to HC or asymptomatic asbestos-exposed (AEx) subjects. We looked for studies using breath analysis as a diagnostic, monitoring and/or prognostic tool comparing the efficacy with the histopathological examination of a biopsy specimen. We also searched the literature for significantly higher breath levels of VOCs, inorganic compounds and fractions of EBC in subjects with MPM and/or asbestosis to differentiate them from healthy (asbestos-exposed) controls, since these non-invasive biomarkers could be used to screen these at-risk groups for MPM.
The databases MEDLINE (PubMed Database) and Web of Science were consulted from September 26, 2017 till March 7, 2018. The following combination of terms was used in both databases: (mesothelioma OR asbestosis OR pleural plaques OR asbestos) AND (breath analysis OR breath test OR volatile organic compounds OR exhaled breath OR exhaled breath condensate). The selection procedure of articles for systematic review is shown in Figure 1.
Flowchart.
In- and exclusion criteria
Using this search strategy, 204 articles were identified. First, duplicates from both databases were removed. Second, the titles and abstracts of the remaining articles were assessed by four independent reviewers (J Vandersnickt, C Millevert, L Arnouts and L Brusselmans) and the outcome compared. If there were disagreements, it was discussed until consensus. We excluded 127 articles based on their title or abstract, and from the 23 remaining articles the full text was read independently by the reviewers. Afterwards, 11 more articles were excluded: 3 had no relevant outcomes and 8 were congress abstracts. Ultimately, 12 articles remained for discussion.
Publication date was not an exclusion criterion, since breath analysis related to this topic is a recent research field (the oldest article dates from 2006). Furthermore, there was no language bias since the keywords we entered into the databases only yielded articles written in English. Reviews were excluded and only primary studies included, focusing on breath analysis as a diagnostic/screening tool and dealing with the pathologies of MPM or benign ARD, like asbestosis. Furthermore, we only included articles on pleural mesothelioma, and other forms as pericardial, peritoneal and tunica vaginalis mesothelioma were excluded. In addition, articles describing breath analysis as a diagnostic tool for other cancers or diseases were excluded. Because the limited research currently available on these topics, we did not screen on age, smoking behaviour or other possible criteria of participants.
Data collection and analysis
The qualitative and quantitative characteristics of the included articles were independently extracted by four reviewers according to the recommendations of the Cochrane Collaboration for diagnostic research (66).
Results and discussion
Twelve relevant studies concerning breath analysis as a diagnostic or screening tool for ARD were included. Table 2 summarizes specific exclusion criteria for the selection of groups together with the different characteristics and the results of the individual studies. In general, the HC included in all the studies, were never occupationally exposed to asbestos, and the research groups used different analyzing instruments, in- and exclusion criteria and patient and control groups. Also the sample size of the studies fluctuates. Because of this heterogeneity, it was not possible to yet run a meta-analysis.

Full table
Promising results were found comparing the different diagnostic tools to distinguish patient and control groups. When looking at the possibility to discriminate HC and MPM patients based upon VOCs, the lowest accuracy was found using MCC-IMS (65%) (31). In contrast, the highest accuracy (95%) was seen using an eNose (73). GC-MS generated intermediate results with an accuracy of 71% (75). Despite the possibility to distinguish MPM patients from HC controls, it is of less clinical importance given that HC persons are not the at-risk groups of interest for screening. More important is thus to look for markers for asbestos exposure by comparing HC with AEx or ARD persons and to look for biomarkers of MPM after asbestos exposure, comparing AEx and/or ARD persons with MPM patients. We found three qualitative studies from the same research group that directly compared HC with AEx subjects in order to identify volatile markers for asbestos-exposure (31,52,75). Using MCC-IMS, these groups could be distinguished with 61–91% accuracy (52,75). Using GC-MS or eNose, the accuracy was respectively 71% and 65% (31).
Since asymptomatic AEx persons and persons with ARD are at the highest risk of developing MPM, these are the groups of interest where a breath test could be used as screening tool. When discriminating MPM patients from AEx subjects, the eNose showed an accuracy ranging from 73% to 81% (74,75), while GC-MS could separate these groups with an accuracy of 97% (31) and MCC-IMS with an accuracy of 88% (52,75). When distinguishing MPM patients from subjects with ARD, the studies yielded an accuracy of 70% using the eNose, 82% using MCC-IMS and 79% using GC-MS (31,75). Furthermore, if both AEx and ARD groups are pooled into one asbestos-exposed group and tried to be discriminated from MPM patients, we find once more the lowest accuracy with eNose (74%) and the highest with GC-MS (94%) (31,75). MCC-IMS analysis discriminated these groups with 85% accuracy (75). This could be explained by the fact that eNoses recognize a pattern of VOCs and do not identify VOCs, in contrast to GC-MS or MCC-IMS, which can focus on specific VOCs. With GC-MS, de Gennaro et al. found cyclopentane as a marker for long-term asbestos exposure and cyclohexane to be the only compound that distinguished MPM patients from former asbestos workers and HC (72). The latter compound was also found by Lamote et al. to distinguish MPM patients from AEx subjects (31), underlining its potential importance to be used as screening biomarker in AEx subjects. Nevertheless, cyclohexane is also identified in animal models with pneumonia (76) and in the breath of lung cancer patients (51). This suggests the VOC is generated by oxidative stress in inflamed tissue and serves as non-specific marker for inflammation, which further explains the lack of specificity for MPM (51). Despite the limited amount of studies, it is remarkable that de Gennaro et al. (72) and Lamote et al. (31) obtained overlapping results regarding VOCs, like cyclopentane, cyclohexane and limonene when using GC-MS. Despite GC-MS being an expensive and time-consuming technique, building targeted sensors sensitive for these specific identified compounds might be a future step in the development of a handheld screening tool.
Next to studies focussing on volatile biomarkers in breath, we also included articles looking at markers in EBC and inorganic compounds in breath (68). Levels of 8-isoprostane were found significantly higher in patients with asbestosis, in AEx subjects and in AEx subjects with borderline parenchymal changes compared to HC (P=0.0001–0.0480) (32,67,68,70). In addition, alveolar NO, was significantly higher in patients with asbestosis and in AEx subjects with borderline parenchymal changes in comparison to HC (P=0.006–0.009) (67,68,70,71). This was also the case with levels of C-reactive protein, interleukine-6 and myeloperoxidase (74). Moreover, leukotriene B4 was the only biomarker that was significantly higher in AEx subjects with normal parenchymal changes compared to HC (P≤0.001) (70).
Furthermore, the levels 8-iso-PGF2α, o-Tyr and 8-OHdG were significantly higher in subjects with asbestosis or silicosis in contrast with HC (P=0.01–0.05) (69). Lastly, Chow et al. found that hydrogen peroxide was significantly higher in patients with asbestosis in comparison with HC (P≤0.05) (68). Despite important findings, the abovementioned biomarkers measure chronic inflammation, and, therefore, are not specific for ARD or MPM. This is also true for alveolar NO, since this compound is also raised in patients with asthma or COPD (67), and for that reason, patients with asthma or COPD were excluded in several studies (67,68,70). Besides NO, also levels of leukotriene B4, produced by activated neutrophils, were found increased in patients with asthma and COPD (67). However, their use to detect MPM is not yet investigated and holds promise for further research.
Diagnostic tools
Three studies used an eNose as a diagnostic tool. This tool recognizes the bulk of the breath (52,73,75) but does not identify the specific VOCs that are responsible for any difference in exhaled breath patterns. This is reflected by lower discriminating characteristics due to a lower specificity, so more investigation is needed to gain more insight and to improve the methodology in the future (73). Nevertheless, it is a very promising and easy to use screening tool, and its discriminating capacity can be increased if specific VOCs of interest can first be identified where after specific orientated sensors can be developed. The group of Lamote et al. is the only one using MCC-IMS as a diagnostic tool, generating clinically relevant results. This is user-friendly, mobile and has low cost with the ability to identify compounds (52,75). A final analyzing technique, GC-MS, is the gold standard of breath tests. It has the highest sensitivity and allows the best identification of compounds, but is very expensive and time-consuming (31). It must be remarked that other promising techniques as SIFT-MS or PTR-MS have not yet been used to investigate their role as tools for MPM.
However, the choice of type of instrument used for breath analysis depends on the characteristics of the disease of interest and the intended use of the breath test. If using a breath test as screening tool for a rare disease as MPM, in a large at risk group such as AEx or ARD subjects, it is first important that the analysis technique yields a high sensitivity (75). In that way, breath tests will have very few false negatives and will have a high chance of detecting a rare disease if disease is present. Secondly, when screening a large at-risk target group for a rare disease, these screening tools should have a high negative predictive value (NPV). In that case, a negative test result reflects a very high change that disease is truly absent. This combination of a high sensitivity and NPV allows for an exclusive screening, whereby the large NPV rules out disease in the large number of screenees, and the high sensitivity will detect the rare disease like MPM when present. In this way, detection of MPM could be optimized, not subjecting every AEx person to a CT-scan, making diagnostic work-up more cost-effective. Considering a maximal sensitivity and NPV, Lamote et al. obtained results with high sensitivity (87–94% with MCC-IMS, 75–82% with eNose and 79–100% with GC-MS) and high NPV (83–96% with MCC-IMS, 54–70% with eNose and 80–100% with GC-MS) when specifically discriminating MPM patients from at risk AEx, ARD of combined subjects (31,75). These results underline the capacity of breath analysis as screening tool for subjects at risk for MPM allowing us to rule out MPM (31,75).
In summary, depending on the population, the best discriminative results are obtained with MCC-IMS or GC-MS. In MPM patients versus ARD subjects and MPM patients versus HC, we can see that the values of the accuracy are the highest in the studies that use MCC-IMS as method (75). In contrast, GC-MS gives the best results with the comparison of MPM patients versus at risk AEx subjects and MPM patients versus AEx and ARD subjects. In the latter groups, Lamote et al. demonstrated a sensitivity and NPV of 100% (31). Given the high cost and analyzing time of these instruments, it is important to combine specific sensors against compounds of interest into a handheld eNose device in order to reduce sampling time and cost while maintaining the optimal screening characteristics.
Confounders
Although breath analysis is promising considering acceptable clinically relevant outcome and sampling advantages, there are some limitations to take into account for the detection of MPM and/or asbestosis. First of all, despite there is no correlation between the degree of asbestos exposure and pleural and parenchymal changes that can be seen on high resolution CT (70), the type of ARD and a wider range of asbestos disorders seem to affect exhaled breath biomarkers (73). Secondly, we cannot rule out or correct for the background asbestos exposure, which is also present in HC. Thirdly, VOCs can also be exogenous. Hence, there is a possibility of environmental background contamination and that the VOCs found in research are not disease-specific but reflect environmental changes (73). Therefore, correction for background contamination is advisable. Despite the fact that some studies took background samples and corrected for this (31,52,72,75), most studies did not, and the effect of background contamination needs to be further investigated. Also, smoking can be a confounding factor that influences the VOC composition. Therefore, some studies matched the included subjects for smoking status (73). However, smoking is not as important as it would be for the diagnosis of lung cancer, because MPM development is not related to a previous smoking behaviour (73). This is strengthened by the fact that there was no correlation found between smoking and the level of 8-isoprostane and the fact that no smoking-related VOCs, like 2,4-dimethyl-1-heptene and 2,3-dimethylheptane (77), were found to discriminate between the patient and control groups in most of the studies. Furthermore, all studies reported that participants were restricted from smoking for at least 2 hours before EBC or breath collection (32), except in the study of Syslová et al. (69), ruling out potential acute effects of smoking on the breath composition. Nevertheless, there were some smoking-related VOCs found in some studies like benzene, 2,5-dimethylfuran and toluene, but these VOCs were not found important discriminators between AEx subjects and MPM patients (31). We also need to take into account that smoking can induce CYP450, which can degrade the VOCs. Because of these reasons, the impact of smoking status on the results is expected to be minimal but the effect of smoking behaviour or VOC composition should be further investigated (31,75).
Fourthly, the concentrations of exhaled breath biomarkers are sometimes difficult to interpret because of the inter-individual biological variability (large coefficients of variance) and different statistical methods used (32). For example, the production of inflammatory mediators can be determined by the individual differences in immune responses to the presence of asbestos fibres. This difference in immune response can influence the susceptibility to develop asbestos related diseases (70).
Fifthly, it seems difficult to match the patient and controls for age. MPM patients are significantly older than HC controls. The reasons for this mismatch are the latency period between first exposure to the causal agent and the diagnosis of this disease and the fact that older HC without significant comorbidities are hard to find (31). Furthermore, patients with asbestos-related disorders are older and consequently have, just like the HC, often concurrent respiratory and other pathologies (71). In general, there is a disagreement about the relevance of age and its effect on their metabolism and VOCs production (75).
Finally, it has to be mentioned that most studies are pilot studies with a cross-sectional design, MPM is a rather rare disease and the number of subjects included in the studies was therefore small (72-74). These small-scale studies show very promising results, but these outcomes need to be validated for larger studies. Hence, it is likely that the data was overfitted and results are overoptimistic. However, the different statistical analysis and validation seemed to be sufficient to obtain a clear separation between breath prints of the different groups (74) and encouraging VOCs were found important biomarkers for further investigation (72).
Nevertheless, the last publication of Lamote et al. (75) had already an increased number of subjects (n=330) among which 52 MPM patients were included. The results were in line with the previous studies and very satisfying, with high numbers of sensitivity and NPV. Unfortunately, it was not sufficient to discriminate between the different stages of MPM due to the low prevalence of sarcomatoid MPM (74). Most studies were pilot studies trying to identify VOCs specific for MPM (73). In these studies, a histological confirmation was needed, and studies were not blinded (31). A major drawback is that none of the studies could investigate the discrimination of asbestos-exposed individuals from early stage MPM patients, since the latter are hard to find. In order to overcome these drawbacks, we suggest future studies to adhere a prospective study design in which AEx subjects are screened and followed-up over time (73) and the investigator is blinded for the underlying pathology (31). This will also allow to study the use of a breath test to compare the different stages of MPM and to screen patients preferable in early stages of the disease, in hope to improve outcome (74).
Conclusions
MPM is a very aggressive cancer mainly caused by a historical exposure to asbestos fibres and, although remaining a rare disease, its incidence is still increasing worldwide. It is a disease that is generally diagnosed at an advanced stage resulting in a high mortality rate. An early diagnosis is assumed to improve patients’ survival and urges the need for a good screening tool. Nowadays, the diagnosis is made with imaging and invasive methods (CT scan, biopsy) due to a lack of non-invasive tools. As described in this review, the results of previous research into breath analysis are very promising. Breath analysis is a non-invasive and easy-to-use tool allowing a discrimination of at-risk groups from MPM patients with VOCs and markers like cyclohexane, 8-isoprostane, 8-OHdG and diethyl ether. The next step is the external validation of the breath compounds as biomarkers for disease and providing a biological link between VOCs of interest and MPM pathogenesis. These latter can be used to exclude MPM in individuals that are exposed to asbestos fibres thereby selecting patients for additional, more invasive diagnostic procedures. It is advised to perform blinded, prospective, case-control studies following up asbestos-exposed subjects over time. This will assess the clinical utility of a breath test and see if MPM can be detected in early stages, in comparison to other lung diseases.
Acknowledgments
Funding: This study was funded by grants from “Kom op tegen Kanker (Stand up to Cancer), the Flemish cancer society” [grant number 2016/10710/2576, grant number 2017/10986/2653].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bonifazi G, Capobianco G, Serranti S. Asbestos containing materials detection and classification by the use of hyperspectral imaging. J Hazard Mater 2018;344:981-93. [Crossref] [PubMed]
- Marsh GM, Riordan AS, Keeton KA, et al. Non-occupational exposure to asbestos and risk of pleural mesothelioma: review and meta-analysis. Occup Environ Med 2017;74:838-46. [Crossref] [PubMed]
- WHO. Asbestos: elimination of asbestos-related diseases. 2017.
- Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol 2008;38 Suppl 1:49-73. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-307. [PubMed]
- Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998;157:1666-80. [Crossref] [PubMed]
- Prazakova S, Thomas PS, Sandrini A, et al. Asbestos and the lung in the 21st century: an update. Clin Respir J 2014;8:1-10. [Crossref] [PubMed]
- Ndlovu N, Rees D, Murray J, et al. Asbestos-related diseases in mineworkers: a clinicopathological study. ERJ Open Res 2017.3. [PubMed]
- O'Reilly KM, McLaughlin AM, Beckett WS, et al. Asbestos-related lung disease. Am Fam Physician 2007;75:683-8. [PubMed]
- Norbet C, Joseph A, Rossi SS, et al. Asbestos-related lung disease: a pictorial review. Curr Probl Diagn Radiol 2015;44:371-82. [Crossref] [PubMed]
- Greillier L, Astoul P. Mesothelioma and asbestos-related pleural diseases. Respiration 2008;76:1-15. [Crossref] [PubMed]
- Schneider F, Sporn TA, Roggli VL. Asbestos fiber content of lungs with diffuse interstitial fibrosis: An analytical scanning electron microscopic analysis of 249 cases. Arch Pathol Lab Med 2010;134:457-61. [PubMed]
- Lemjabbar-Alaoui H, Hassan OU, Yang YW, et al. Lung cancer: Biology and treatment options. Biochim Biophys Acta 2015;1856:189-210. [PubMed]
- Marinaccio A, Binazzi A, Cauzillo G, et al. Epidemiological surveillance of malignant mesothelioma cases in Italy: incidence and asbestos exposure figures by the Italian mesothelioma registry (ReNaM). Epidemiol Prev 2007;31:23-6. [PubMed]
- Røe OD, Stella GM. Malignant pleural mesothelioma: history, controversy and future of a manmade epidemic. Eur Respir Rev 2015;24:115-31. [Crossref] [PubMed]
- Bianchi C, Bianchi T. Global mesothelioma epidemic: Trend and features. Indian J Occup Environ Med 2014;18:82-8. [Crossref] [PubMed]
- Belgian Cancer Registry. Belgium: Males, number of invasive tumours by primary site and age group in 2015. Available online: kankerregister.org
- Belgian Cancer Registry. Belgium: Females, number of invasive tumours by primary site and age group in 2015. Available online: kankerregister.org
- Kameda T, Takahashi K, Kim R, et al. Asbestos: use, bans and disease burden in Europe. Bull World Health Organ 2014;92:790-7. [Crossref] [PubMed]
- Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer 2007;43:2722-8. [Crossref] [PubMed]
- Kent M, Rice D, Flores R. Diagnosis, staging, and surgical treatment of malignant pleural mesothelioma. Curr Treat Options Oncol 2008;9:158-70. [Crossref] [PubMed]
- Soeberg MJ, Leigh J, Driscoll T, et al. Incidence and survival trends for malignant pleural and peritoneal mesothelioma, Australia, 1982-2009. Occup Environ Med 2016;73:187-94. [Crossref] [PubMed]
- Mazurek JM, Syamlal G, Wood JM, et al. Malignant Mesothelioma Mortality - United States, 1999-2015. MMWR Morb Mortal Wkly Rep 2017;66:214-8. [Crossref] [PubMed]
- Fintelmann FJ, Gottumukkala RV, McDermott S, et al. Lung Cancer Screening: Why, When, and How? Radiol Clin North Am 2017;55:1163-81. [Crossref] [PubMed]
- Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005;366:397-408. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018. [Epub ahead of print]. [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Bonelli MA, Fumarola C, La Monica S, et al. New therapeutic strategies for malignant pleural mesothelioma. Biochem Pharmacol 2017;123:8-18. [Crossref] [PubMed]
- Marcq E, Pauwels P, van Meerbeeck JP, et al. Targeting immune checkpoints: New opportunity for mesothelioma treatment? Cancer Treat Rev 2015;41:914-24. [Crossref] [PubMed]
- Kondola S, Manners D, Nowak AK. Malignant pleural mesothelioma: an update on diagnosis and treatment options. Ther Adv Respir Dis 2016;10:275-88. [Crossref] [PubMed]
- Lamote K, Brinkman P, Vandermeersch L, et al. Breath analysis by gas chromatography-mass spectrometry and electronic nose to screen for pleural mesothelioma: a cross-sectional case-control study. Oncotarget 2017;8:91593-602. [Crossref] [PubMed]
- Pelclová D, Fenclova Z, Kacer P, et al. Increased 8-isoprostane, a marker of oxidative stress in exhaled breath condensate in subjects with asbestos exposure. Ind Health 2008;46:484-9. [Crossref] [PubMed]
- Lagniau S, Lamote K, van Meerbeeck JP, et al. Biomarkers for early diagnosis of malignant mesothelioma: Do we need another moonshot? Oncotarget 2017;8:53751-62. [Crossref] [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian consensus conference on malignant pleural mesothelioma: state of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [Crossref] [PubMed]
- Roggli VL, Gibbs AR, Attanoos R, et al. Pathology of asbestosis- An update of the diagnostic criteria: Report of the asbestosis committee of the college of american pathologists and pulmonary pathology society. Arch Pathol Lab Med 2010;134:462-80. [PubMed]
- Creaney J, Robinson BWS. Malignant Mesothelioma Biomarkers: From Discovery to Use in Clinical Practice for Diagnosis, Monitoring, Screening, and Treatment. Chest 2017;152:143-9. [Crossref] [PubMed]
- Demir M, Kaya H, Taylan M, et al. Evaluation of New Biomarkers in the Prediction of Malignant Mesothelioma in Subjects with Environmental Asbestos Exposure. Lung 2016;194:409-17. [Crossref] [PubMed]
- Sun HH, Vaynblat A, Pass HI. Diagnosis and prognosis-review of biomarkers for mesothelioma. Ann Transl Med 2017;5:244. [Crossref] [PubMed]
- Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol 2008;3:1317-24. [Crossref] [PubMed]
- Bonotti A, Foddis R, Landi S, et al. A novel panel of serum biomarkers for MPM diagnosis. Dis Markers 2017;2017. [Crossref] [PubMed]
- Bonotti A, Simonini S, Pantani E, et al. Serum mesothelin, osteopontin and vimentin: useful markers for clinical monitoring of malignant pleural mesothelioma. Int J Biol Markers 2017;32:e126-31. [Crossref] [PubMed]
- Cui A, Jin XG, Zhai K, et al. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open 2014;4. [Crossref] [PubMed]
- Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med 2012;367:1417-27. [Crossref] [PubMed]
- Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Clin Cancer Res 2016;22:3087-96. [Crossref] [PubMed]
- Boots AW, van Berkel JJ, Dallinga JW, et al. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res 2012;6. [Crossref] [PubMed]
- Romano A, Capozzi V, Spano G, et al. Proton transfer reaction-mass spectrometry: online and rapid determination of volatile organic compounds of microbial origin. Appl Microbiol Biotechnol 2015;99:3787-95. [Crossref] [PubMed]
- Haick H, Broza YY, Mochalski P, et al. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 2014;43:1423-49. [Crossref] [PubMed]
- Smith D, Spanel P. Ambient analysis of trace compounds in gaseous media by SIFT-MS. Analyst 2011;136:2009-32. [Crossref] [PubMed]
- Pleil JD, Wallace A, Madden MC. Exhaled breath aerosol (EBA): the simplest non-invasive medium for public health and occupational exposure biomonitoring. J Breath Res 2018;12. [Crossref] [PubMed]
- Winters BR, Pleil JD, Angrish MM, et al. Standardization of the collection of exhaled breath condensate and exhaled breath aerosol using a feedback regulated sampling device. J Breath Res 2017;11. [Crossref] [PubMed]
- Lamote K, Nackaerts K, van Meerbeeck JP. Strengths, weaknesses, and opportunities of diagnostic breathomics in pleural mesothelioma-a hypothesis. Cancer Epidemiol Biomarkers Prev 2014;23:898-908. [Crossref] [PubMed]
- Lamote K, Vynck M, Van Cleemput J, et al. Detection of malignant pleural mesothelioma in exhaled breath by multicapillary column/ion mobility spectrometry (MCC/IMS). J Breath Res 2016;10. [Crossref] [PubMed]
- Jiménez-Pacheco A, Salinero-Bachiller M, Iribar MC, et al. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Markar SR, Chin ST, Romano A, et al. Breath Volatile Organic Compound Profiling of Colorectal Cancer Using Selected Ion Flow-Tube Mass Spectrometry. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Tong H, Wang Y, Li Y, et al. Volatile organic metabolites identify patients with gastric carcinoma, gastric ulcer, or gastritis and control patients. Cancer Cell Int 2017;17:108. [Crossref] [PubMed]
- Kataoka H, Saito K, Kato H, et al. Noninvasive analysis of volatile biomarkers in human emanations for health and early disease diagnosis. Bioanalysis 2013;5:1443-59. [Crossref] [PubMed]
- Jareño-Esteban JJ, Munoz-Lucas MA, Gomez-Martin O, et al. Study of 5 Volatile Organic Compounds in Exhaled Breath in Chronic Obstructive Pulmonary Disease. Arch Bronconeumol 2017;53:251-6. [Crossref] [PubMed]
- van Oort PM, Povoa P, Schnabel R, et al. The potential role of exhaled breath analysis in the diagnostic process of pneumonia-a systematic review. J Breath Res 2018;12. [Crossref] [PubMed]
- Ulanowska A, Kowalkowski T, Hrynkiewicz K, et al. Determination of volatile organic compounds in human breath for Helicobacter pylori detection by SPME-GC/MS. Biomed Chromatogr 2011;25:391-7. [Crossref] [PubMed]
- Peng G, Hakim M, Broza YY, et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer 2010;103:542-51. [Crossref] [PubMed]
- Neerincx AH, Vijverberg SJH, Bos LDJ, et al. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr Pulmonol 2017;52:1616-27. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Shende P, Vaidya J, Kulkarni YA, et al. Systematic approaches for biodiagnostics using exhaled air. J Control Release 2017;268:282-95. [Crossref] [PubMed]
- Storer M, Curry K, Squire M, et al. Breath testing and personal exposure--SIFT-MS detection of breath acetonitrile for exposure monitoring. J Breath Res 2015;9. [Crossref] [PubMed]
- Smith D, Spanel P. SIFT-MS and FA-MS methods for ambient gas phase analysis: developments and applications in the UK. Analyst 2015;140:2573-91. [Crossref] [PubMed]
- Collaboration TC. Cochrane Belgium 2017. Available online: http://belgium.cochrane.org/en/contact-us
- Lehtonen H, Oksa P, Lehtimaki L, et al. Increased alveolar nitric oxide concentration and high levels of leukotriene B(4) and 8-isoprostane in exhaled breath condensate in patients with asbestosis. Thorax 2007;62:602-7. [Crossref] [PubMed]
- Chow S, Campbell C, Sandrini A, et al. Exhaled breath condensate biomarkers in asbestos-related lung disorders. Respir Med 2009;103:1091-7. [Crossref] [PubMed]
- Syslová K, Kacer P, Kuzma M, et al. LC-ESI-MS/MS method for oxidative stress multimarker screening in the exhaled breath condensate of asbestosis/silicosis patients. J Breath Res 2010;4. [Crossref] [PubMed]
- Lehtimäki L, Oksa P, Jarvenpaa R, et al. Pulmonary inflammation in asbestos-exposed subjects with borderline parenchymal changes on HRCT. Respir Med 2010;104:1042-9. [Crossref] [PubMed]
- Sandrini A, Johnson AR, Thomas PS, et al. Fractional exhaled nitric oxide concentration is increased in asbestosis and pleural plaques. Respirology 2006;11:325-9. [Crossref] [PubMed]
- de Gennaro G, Dragonieri S, Longobardi F, et al. Chemical characterization of exhaled breath to differentiate between patients with malignant plueral mesothelioma from subjects with similar professional asbestos exposure. Anal Bioanal Chem 2010;398:3043-50. [Crossref] [PubMed]
- Chapman EA, Thomas PS, Stone E, et al. A breath test for malignant mesothelioma using an electronic nose. Eur Respir J 2012;40:448-54. [Crossref] [PubMed]
- Dragonieri S, van der Schee MP, Massaro T, et al. An electronic nose distinguishes exhaled breath of patients with Malignant Pleural Mesothelioma from controls. Lung Cancer 2012;75:326-31. [Crossref] [PubMed]
- Lamote K, Vynck M, Thas O, et al. Exhaled breath to screen for malignant pleural mesothelioma: a validation study. Eur Respir J 2017.50. [PubMed]
- Zhou Y, Chen E, Wu X, et al. Rational lung tissue and animal models for rapid breath tests to determine pneumonia and pathogens. Am J Transl Res 2017;9:5116-26. [PubMed]
- Chen X, Wang F, Lin L, et al. Association of Smoking with Metabolic Volatile Organic Compounds in Exhaled Breath. Int J Mol Sci 2017.18. [PubMed]