Appropriate screening intervals in low-dose CT lung cancer screening
Introduction
One of the pending challenges in the optimization of lung cancer screening by low-dose chest CT (LDCT) is the definition of the best screening regime. Currently, lung cancer screening is being implemented in routine clinical care in the United States, and other countries may follow in near future (1). The US Preventive Services Task Force recommends annual lung cancer screening by LDCT in high-risk individuals (aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years), based on the results of the National Lung Screening Trial (NLST) (2,3). This largest randomized-controlled LDCT lung cancer screening trial worldwide demonstrated a 20% decrease in lung cancer specific mortality in the intervention group, screened by three annual LDCTs, compared to the control group, screened by three annual chest radiographs (3). As a result of these findings, current lung cancer screening programmes include up to 25 annual LDCTs plus additional short-term follow-up LDCTs when indicated. However, the choice of a yearly CT scan has not been based on biological mechanisms, and it is questionable whether all persons eligible for lung cancer screening require annual screening (4). Recently, more and more evidence for a more personalized screening regime has become available. Selection of eligible participants at highest risk of lung cancer detection by a risk-prediction model has been shown to lead to more efficient lung cancer screening with higher cumulative lung cancer incidence and more early stage lung cancers detected (5). It is hypothesized that adding lung cancer risk based on LDCT characteristics to lung cancer risk of participant already enrolled in a screening program could help to identify a subset of participants at lower lung cancer risk based on their baseline screening CT who can safely be followed by a prolonged screening interval. This will improve the benefit-to-harm ratio and will lead to more efficient lung cancer screening programs. The aim of this review is to discuss current evidence on optimal screening intervals in LDCT lung cancer screening.
Results from lung cancer screening studies
Early-stage lung cancer mostly presents as a small solid lung nodule. In about 50% of lung cancer screening participants, at least one pulmonary nodule is found at the baseline examination, and around a quarter of screenees have at least two lung nodules (6). The large majority of these nodules are benign, and only nodules above a certain size cut-off need additional follow-up. This leaves a substantial part of screening participants with a negative baseline screening. Both researchers from the NLST as well as the Dutch-Belgian randomized-controlled lung cancer screening (Dutch acronym: NELSON) trial have retrospectively evaluated lung cancer risk in screening participants based on their (negative) baseline screen result. In a third randomized-controlled lung cancer screening trial, the Multicentric Italian Lung Detection (MILD) trial, annual and biannual screening strategies were compared prospectively.
MILD trial
The only randomized-controlled lung cancer screening trial in which different screening intervals were used in the randomization of screen participants was the Italian MILD trial. In total, 4,099 participants were randomized to annual screening (n=1,190), biannual screening (n=1,186), or no screening (control group, n=1,723). Participants were current or former heavy smokers, at least 49 years old, who smoked at least 20 pack-years and quitted maximum 10 years before recruitment. In this trial, no difference in lung cancer specific mortality was found between the different study arms, although the study had not been sufficiently powered to show such a difference (7).
In a post-hoc analysis, performance between annual and biannual screening was compared (8). Lung cancer detection rate for annual and biannual screening did not significantly differ (3.6% versus 2.7%, respectively). Moreover, the percentage of early stage lung cancers was comparable between the two screening regimes (53.6% versus 59.2% stage I, and 26.8% versus 22.2% stage IV, respectively), and a comparable distribution of screen-detected and interval cancers was found (69.0% versus 67.7%, respectively).
In the MILD trial, sensitivity, specificity, positive predictive value, negative predictive value, and mortality rates were similar for annual and biannual screening. This indicates that a 2-year screening interval may be at least as efficient as annual screening (8), although this study was limited by a relatively small sample size and possibly biased by participants randomized to biannual screening with abnormalities on their baseline CT and therefore moving to annual (follow-up) screening.
NLST
Recently, Patz et al. performed a retrospective cohort analysis of data from the NLST (9). The NLST was a multi-center randomized controlled lung cancer screening trial in which 53,454 participants (aged 55–74 years, current or former heavy smokers with at least a 30 pack-year history, and if a former smoker, had quitted less than 15 years before study entry) were randomized (1:1) to three annual low-dose CT screenings or three annual chest radiographies (3). Participants with a negative baseline screen, defined as no nodules or largest nodule <4 mm and no abnormal mediastinal or pleural findings, were invited for the regular next annual screen. Patz et al. determined lung cancer incidence and mortality in all participants who underwent a baseline (T0) screening, and compared this with participants with a negative baseline screen result. They aimed to evaluate the value of an annual screen after a negative baseline screening result.
In total, 26,231 participants underwent a baseline screen, of which 19,066 (73%) had a negative baseline screen. Over all three screening rounds, 14,686 participants (56%) had only negative screen results. Four per cent of participants (n=1,063) who underwent the baseline screening were eventually diagnosed with lung cancer, maximum 5 years after the last screening round. Of the 19,066 participants with a negative baseline screening round, 441 (2%) were eventually diagnosed with lung cancer. Ninety-two of these participants (0.48%) had lung cancer diagnosed within 2 years after the baseline screening (30 interval cancers, 62 screen-detected cancers; 52% stage I or II), and an additional 118 participants had lung cancer detected at or <1 year after the third screening 2 years after baseline (60% stage I or II). Because of the low lung cancer incidence in the initial annual screening round in participants with a negative baseline screen, compared to those with a positive baseline screen, it was suggested that annual screening after a negative screen might be unnecessary. The overall incidence of lung cancer and lung cancer mortality in participants with a negative baseline screen (371.9 per 100,000 observed patient-years and 277.2 per 100,000 observed patient-years, respectively) was lower than that for all participants who underwent a baseline screen (661.2 per 100,000 observed patient years, and 185.8 per 100,000 observed patient years, respectively). Participants with three negative screenings had significantly lower lung cancer incidence and mortality compared to all participants who underwent a baseline screening (9).
The reason for the relatively low risk of lung cancer death in participants with a negative baseline screening is unknown. Patz et al. suggest that these participants may have relatively slow growing—indolent—cancers or may have lungs that are less sensitive to the harmful effects of smoking. However, results on new nodules detected after baseline call into question the first explanation. In participants with a negative baseline screening, lung cancer diagnosis at incidence screening rounds is often made in newly developed nodules (10,11). These nodules are known for their fast growth and high cancer rate. Lung cancer in new nodules has been found to be less often early stage after a biannual screening interval when compared to an annual screening interval (64% versus 79% stage I, respectively), not taking into account the result of the baseline screening round (10). This questions the first explanation as suggested by Patz et al.
NELSON trial
In the NELSON trial, 15,792 participants were randomized to either screening by LDCT (n=7,900) or no screening. Eligible participants were current or former (quit duration maximum 10 years before study enrolment) heavy smokers who smoked >15 cigarettes per day for >25 years or >10 cigarettes per day for >30 years, between 50 and 75 years old. To evaluate the effect of prolonged screening intervals, participants randomized to the LDCT arm were screened at baseline (year 1), 1 year later (year 2, annual screening), in year 4 (biannual screening), and in year 6.5 (2.5 years screening interval).
When assessing lung cancer probability 2 years after baseline, it was found that participants with a negative baseline screening (newly proposed cut-off volume of largest nodule <100 mm3) had comparable low risk of developing lung cancer during the next 2 years as participants without any baseline nodules (0.6% versus 0.4%, respectively) (12). For these participants, 2-year lung cancer probability was significantly lower compared to participants with an intermediate-risk (100–300 mm3, 2-year lung cancer probability 2.4%) or high-risk nodule (>300 mm3, 2-year lung cancer probability 16.9%) at baseline, suggesting that an annual LDCT after a negative baseline screen might not be necessary.
When assessing lung cancer risk in NELSON participants based on their previous screening history, it was found that a participant’s screening history can be used as a risk stratification tool for their further screening regime (13), like the findings by Patz et al. (9). The risk of lung cancer diagnosis in the fourth screening round, 2.5 years after round 3, differed significantly between participants with a negative (lung cancer risk 0.6%) and indeterminate (lung cancer risk 3.7%) third round result. Few participants with a positive third screening round result participated in the fourth screening round, none of them was diagnosed with lung cancer. Next to screening history, older age and higher pack-years were found to be significant predictors for a non-negative screen result in the fourth screening round. The latter are already used as risk factors for participant selection in lung cancer screening programs.
In the NELSON study, the number of interval cancers has been shown to substantially rise after a prolonged screening interval. In the period between the baseline LDCT in year 1 and the second screening round in year 2, five interval cancers were detected, all stage IIIB or IV. Between the second and third (year 4) screening round, nineteen interval cancers were detected of which seven in the first year and twelve in the second year after the second screening round. Fourteen of these cancers were detected in stage III or IV (14). A true-negative last screen result was given to 4/12 participants diagnosed with fast-growing interval lung cancer the first 2 years after baseline and to 7/12 participants diagnosed with fast-growing interval lung cancer in the third year after baseline. The other 13 interval cancers where present at the last screening, but not classified as positive due to detection error (n=9) caused by, i.e., pleura-attachment and intrabronchial location, protocol inadequacy (n=1), human error (n=1), or interpretation error (n=2). When determining the optimal screen interval, the period for a lung cancer to develop and progress from a visible nodule (cut-off volume ~15 mm3) to lung cancer beyond early stage could be directional. The length of this period depends on the fastest lung cancer growth rate, expressed in volume doubling time (VDT). Cancers diagnosed in baseline nodules after a negative baseline screening have generally slower VDTs than cancers diagnosed after an indeterminate or positive baseline screening (15,16). On the contrary, lung cancers diagnosed in newly developed nodules usually have short VDTs, with a median estimated VDT of around 100 days (10). New nodule lung cancers with VDTs as fast as 24 days have been described (10), meaning that adding an extra year to a screening interval could lead to 15 extra doublings in nodule volume and most probably missing the chance of early detection of some lung cancers. Increasing the screening interval even more, to a 2.5-year period, has been shown to lead to detection of significantly more interval cancers and fewer early-staged cancers compared to both a one and 2-year screening interval (17).
In summary, the low 2-year lung cancer probability after a negative (largest nodule <100 mm3) baseline screening implies that an annual screen might not be necessary for a subset of screening participants. On the other hand, the number of interval cancers was found to significantly increased in the second year of a biannual screening interval. The most optimal interval for participants with a negative baseline screen result may be neither an annual nor a biannual scan, but somewhere between 1 and 2 years.
Subsolid nodules
In lung cancer screening, subsolid (both pure ground-glass and ground-glass with a solid component) nodules are a special entity. Both in the Early Lung Cancer Action Project (ELCAP), the NLST and the NELSON study, it was found that although subsolid nodules possess a high lung cancer probability, they very rarely lead to lung cancer death (18-20). Even in the case of a new subsolid nodule, the chance of lung cancer mortality caused by such a nodule is very low (18,21,22). Therefore, it is suggested that these nodules can be followed safely after a prolonged screening interval, if they have been proven to be stable at short-term follow up LDCT (18,19,23). Thus, for screening participants with a baseline or new subsolid nodule of any size and without higher risk solid nodules, a short-term follow-up LDCT is recommended (1). In case the subsolid nodule remains stable and no new solid nodules or growing small-sized baseline nodules are present, a prolonged screening interval could be considered (Table 1). Again, no biological evidence exists for the length of this prolonged screening interval; the optimum probably lies between 1 and 2 years.
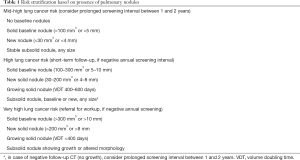
Full table
Selection of the optimal screening interval based on lung cancer risk
To be eligible for lung cancer screening, participants must be above a certain risk for lung cancer development in the near future. Selection of participants using a risk-prediction tool, including other risk factors like family history of lung cancer and clinical diagnosis of COPD, helps to identify individuals at highest lung cancer risk (24,25). This has been shown to improve the efficacy of a screening program (5,26).
An individual’s risk, based on age and number of smoked pack-years, does in principle not change after a participant has undergone the baseline screening. Lung cancer risk may only reduce over time in case a former smoker does not develop lung cancer during screening, until the moment he is not eligible for screening anymore as he has quit smoking for more than 10 years. Therefore, varying the screening regime between eligible high-risk participants may seem illogical. In absolute terms, a prolonged screen interval will be less effective also for a group at mid-high lung cancer risk as it will potentially lead to more late-staged screen-detected cancers and more interval cancers. Nevertheless, reducing the number of LDCTs induces substantially fewer harms such as overdiagnosis and work-up for benign diseases, and might therefore be similarly cost-effective (27). By adding LDCT characteristics like presence of pulmonary nodules to risk-prediction models, participants at mid-high 2-year lung cancer risk could be selected. These participants have a significantly lower risk to develop lung cancer during the next 2 years compared to participants at high and very high lung cancer risk derived from their baseline screening result (Table 1). Based on the evidence from the MILD, NLST, and NELSON study, this subset of participants at mid-high 2-year lung cancer risk could be followed using a prolonged screening interval, until a baseline nodule starts growing or they develop a new solid nodule.
Next to LDCT characteristics of pulmonary nodules, presence of CT imaging biomarkers for COPD at the LDCT, expressed in emphysema score and bronchial wall thickness, might be helpful in identification of participants at highest lung cancer risk. Participants with more severe emphysema were found to be at higher risk of lung cancer development. In the Brock model for the assessment of a lung nodule’s cancer probability (28), presence or absence of emphysema on the CT scan as reported by a radiologist is included as an independent predictor. Additional studies have shown a strong correlation between CT imaging biomarkers for COPD and lung cancer diagnosis (29-31). Recently, a study showed that selecting eligible lung cancer screening participants by adding the presence of CT-quantified emphysema to the NLST selection criteria lead to a decreased number needed to screen to select one lung cancer patient (32,33). More knowledge on the relationship of the degree of CT-quantified emphysema and bronchial wall thickness and lung cancer probability and mortality is needed to evaluate its possible role in risk stratification of lung cancer screening participants.
Conclusions
Currently, evidence is available for lung cancer screening by annual LDCT alone. However, based on retrospective analyses of the largest randomized-controlled lung cancer screening trials, a subset of participants with a low 2-year lung cancer probability as extracted from their baseline screen may be safely followed after a prolonged screening interval (optimal screening interval probably between 1 and 2 years) until their risk profile changes. In case a new pulmonary nodule appears at subsequent screening, or a small baseline nodule starts growing, participants should always return to annual LDCT screening after the appropriate workup.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Heuvelmans MA, Oudkerk M. Determination of the optimal screen interval in low-dose CT lung cancer screening: are we there yet? Transl Cancer Res 2016;5:S1070-2. [Crossref]
- Tammemägi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol 2017;18:1523-31. [Crossref] [PubMed]
- Heuvelmans MA, Walter JE, Peters RB, et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer 2017;113:45-50. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- Sverzellati N, Silva M, Calareso G, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26:3821-9. [Crossref] [PubMed]
- Patz EF Jr, Greco E, Gatsonis C, et al. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol 2016;17:590-9. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907-16. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, Oudkerk M. Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl Lung Cancer Res 2017;6:42-51. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax 2017;72:819-24. [Crossref] [PubMed]
- Horeweg N, Scholten E, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [Crossref] [PubMed]
- Heuvelmans MA, Oudkerk M, de Bock GH, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol 2013;23:1836-45. [Crossref] [PubMed]
- Heuvelmans MA, Vliegenthart R, de Koning HJ, et al. Quantification of growth patterns of screen-detected lung cancers: The NELSON study. Lung Cancer 2017;108:48-54. [Crossref] [PubMed]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- Yankelevitz DF, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015;277:555-64. [Crossref] [PubMed]
- Scholten ET, de Jong PA, de Hoop B, et al. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J 2015;45:765-73. [Crossref] [PubMed]
- Yip R, Yankelevitz DF, Hu M, et al. Lung Cancer Deaths in the National Lung Screening Trial Attributed to Nonsolid Nodules. Radiology 2016;281:589-96. [Crossref] [PubMed]
- Henschke CI, Yip R, Smith JP, et al. CT screening for lung cancer: Part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol 2016;207:1176-84. [Crossref] [PubMed]
- Heuvelmans MA, Walter JE, Oudkerk M. Management of baseline and new sub-solid nodules in CT lung cancer screening. Expert Rev Respir Med 2018;12:1-3. [Crossref] [PubMed]
- Heuvelmans MA, Oudkerk M. Management of subsolid pulmonary nodules in CT lung cancer screening. J Thorac Dis 2015;7:1103-6. [PubMed]
- Raji OY, Duffy SW, Agbaje OF, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med 2012;157:242-50. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Field JK, Chen Y, Marcus MW, et al. The contribution of risk prediction models to early detection of lung cancer. J Surg Oncol 2013;108:304-11. [Crossref] [PubMed]
- de Koning HJ. Benefits and harms of computed tomography lung cancer screening strategies: A comparative modeling study for the U.S. Preventive services task force. Ann Intern Med 2014;160:311-20. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Mets OM, Buckens CF, Zanen P, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA 2011;306:1775-81. [Crossref] [PubMed]
- Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012;141:1216-23. [Crossref] [PubMed]
- de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932-8. [Crossref] [PubMed]
- Sanchez-Salcedo P, Wilson DO, De-Torres JP, et al. Improving selection criteria for lung cancer screening: The potential role of emphysema. Am J Respir Crit Care Med 2015;191:924-31. [Crossref] [PubMed]
- de-Torres JP, Marín JM, Casanova C, et al. Identification of COPD Patients at High Risk for Lung Cancer Mortality Using the COPD-LUCSS-DLCO. Chest 2016;149:936-42. [Crossref] [PubMed]


