Shared decision-making conversations and smoking cessation interventions: critical components of low-dose CT lung cancer screening programs
Introduction
Every year, more deaths occur from lung cancer than from colon, breast, and prostate cancers combined. Lung cancer is the most common cancer worldwide, accounting for 1.8 million new cases and 1.6 million deaths in 2012 (1). Lung cancer is often diagnosed at an advanced stage, resulting in a 5-year survival rate of 16% (2). Screening and detection of earlier-stage disease has improved the survival of patients with the aforementioned non-lung cancers; an effective screening method for lung cancer was lacking until recently.
In the words of the lung cancer screening (LCS) and surveillance task force of the American Association for Thoracic Surgery (AATS), “…at this time and for the first time in medical history, we can say, ‘lung cancer screening—the time has come.’” (3). This statement stems from the evidence that annual screening for lung cancer with low-dose computed tomography (LDCT) can reduce lung cancer mortality in high-risk individuals. However, LDCT lung screening is not without harms and patients need to be educated about the potential benefits, harms, and limitations of screening to make informed decisions about LCS.
This review provides: (I) a brief summary of a large randomized controlled trial (RCT) on LDCT LCS that has changed the screening landscape; (II) various LCS guidelines established by professional and medical organizations, with particular attention given to smoking cessation and shared decision-making (SDM); and (III) SDM definitions and evidence of its ability to increase patient-centered care, with tools for SDM. The paper also suggests implementation strategies for incorporating SDM for LCS in clinical settings.
Overview of the evidence for LCS with LDCT
Given the public health burden of lung cancer, an effective screening strategy that detects early-stage disease has been sought for decades. Earlier screening tests, i.e., sputum cytology and chest radiographs, were able to detect small, earlier stage tumors. However, screening with these modalities did not decrease the number of deaths from lung cancer nor the number of advanced lung cancers (4). Thus, they are not recommended for LCS.
RCTs and observational studies have shown that LCS with LDCT reduces deaths from lung cancer (4-6). Two small European trials, the Detection and Screening of Early Lung Cancer by Novel Imaging Technology and Molecular Essays (DANTE) (7,8) and the Danish Lung Cancer Screening Trial (DLCST) (9) were underpowered enrolling 2,472 and 4,104 individuals, respectively, and considered of fair-quality. Another European trial, the Multicentric Italian Lung Detection (MILD), is considered of poor quality due to concerns about the adequacy of randomization (10). The National Lung Screening Trial (NLST), published in 2011, is the largest RCT and showed LDCT for LCS reduced lung cancer-related deaths by 20% (11). The NLST randomized 53,454 current and former smokers to three annual screenings (baseline, 1 year and 2 years after baseline) with LDCT or chest radiography with a median follow-up of 6.5 years. The NLST had 90% power to detect a 21% decrease in lung cancer-specific mortality in the LDCT group compared to the control group. Greater reduction in lung cancer-specific mortality with LDCT may have been seen if the control group received no screening or if annual LDCT examinations continued beyond 3 years.
To save one life, about 320 people would need to be screened based on the NLST data (11). In the systematic review on the benefits and harms of LDCT LCS by Bach et al., the authors conclude that “screening a population of individuals at a substantially elevated risk of lung cancer most likely could be performed in a manner such that the benefits that accrue to a few individuals outweigh the harms that many will experience.” (4). This statement attests to the fact that LCS is not without harms, such as false-positive results, which can lead to invasive procedures and potential complications (4,6). In the NLST, 96.4% of positive screening results were false positives, and most cases were resolved with one follow-up LDCT scan (12). Only about 1.9% of NLST participants had to undergo biopsy to determine if abnormalities on imaging identified by screening were in fact cancer (12). Other harms include overdiagnosis (the diagnosis of a cancer that would never have caused harm in a person’s lifetime), overtreatment, false-negative results, incidental findings, and radiation exposure.
Guidelines on LDCT LCS and attention to SDM and smoking cessation
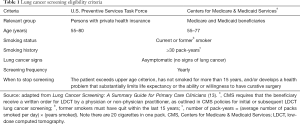
In 2014, the U.S. Preventive Services Task Force (USPSTF) issued a grade B recommendation for annual LCS with LDCT for individuals that meet the criteria listed in Table 1 (14). The USPSTF found that LDCT had a net benefit, albeit moderate, when performed annually in patients who are at high risk for lung cancer based on pack-year smoking history, age, and years since quitting smoking; the available evidence for the efficacy of LDCT screening in decreasing lung cancer mortality was deemed of moderate certainty. Many major medical and professional societies have endorsed LCS with LDCT, including the American Cancer Society (15), American College of Chest Physicians (16,17), American Thoracic Society (16), Society of Thoracic Surgeons (18), American College of Radiology (19), American Society of Clinical Oncology (20), and the American Lung Association (21). The American Academy of Family Physicians concluded there is currently insufficient evidence to recommend for or against screening for lung cancer with LDCT among high-risk individuals (22).
Full table
In 2015, the Centers for Medicare & Medicaid Services (CMS) issued a coverage determination for LCS with LDCT (23). After reviewing relevant clinical evidence and soliciting public comments, CMS found sufficient evidence to add LDCT lung screening as a preventive service benefit under the Medicare program. While annual screening is covered for beneficiaries who meet the criteria outlined in Table 1, an unprecedented prerequisite must occur: an LCS counseling and SDM visit using one or more decision aids. The beneficiary must receive a written order for LDCT LCS during a counseling and SDM visit provided by a physician, physician assistant, nurse practitioner, or clinical nurse specialist. The SDM and counseling visit is coded and reimbursed separately from the annual LDCT LCS to emphasize the importance of patient selection.
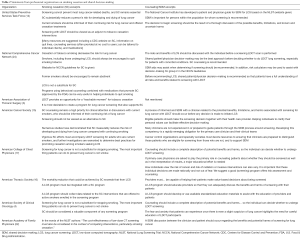
SDM components of various professional organizations are highlighted in Table 2. Benefits of screening need to be discussed, which include early detection of disease and potential reduction in treatment-related morbidity compared to late-stage cancer therapy, and reduced lung cancer-specific mortality. Potential harms must also be addressed which include the high false-positive rate of LDCT and the associated follow-up testing and procedures, overdiagnosis, and radiation exposure. Other harms must be taken into consideration, including anxiety from indeterminate results and the work up of incidental findings (e.g., coronary artery disease and emphysema), and financial concerns. Thus, the SDM process must integrate the knowns and unknowns of lung screening, along with patients’ values, preferences, health and functional status, and eligibility for screening, for them to make informed decisions on LCS.
Full table
The counseling requirements of the visit include emphasizing the commitment to annual LDCT screening until patients no longer meet screening criteria, discussion of patients’ comorbidities and their ability or willingness to undergo diagnosis and treatment, and providing information on smoking cessation. While the initial LDCT lung screening service must include the counseling and SDM visit, subsequent annual screening does not require one.
Although CMS outlines components of the SDM and counseling visit, major uncertainties exist. Specific decision aids were not mentioned and CMS has not issued an “approved” decision aid. Smoking cessation counseling and interventions are emphasized during routine primary care visits as part of the counseling visit before screening, and CMS lists eligibility criteria for imaging facilities to “make available” smoking cessation interventions; however, no specific details are provided on type, intensity or duration of interventions which should be provided.
Guidelines from major organizations also highlight the need for smoking cessation interventions in LCS programs (Table 2). Some guidelines briefly mention smoking cessation, while others like the USPSTF recommendation statement go into greater detail. Smoking cessation is the most effective way of decreasing one’s risk of lung cancer. For instance, Halpern et al. found that with increasing age, adults who quit smoking between the ages of 30 to 49 years had only a slightly higher risk of dying from lung cancer compared to nonsmokers, while those who quit between the ages of 50 to 64 years retained the risk attained at the time of cessation (25). While LDCT LCS is an important means of reducing lung cancer mortality, smoking cessation or continued abstinence for past smokers is of primary importance.
Guidelines vary in eligibility requirements and risk factors for lung cancer. CMS largely utilizes the NLST inclusion criteria to identify eligibility for LCS in high-risk smokers (≥30 pack-year history of smoking, current smokers or recently quit within the past 15 years, no clinical symptoms of lung cancer, age 55–74 years); CMS increased the upper age limit to 77 (23). The National Comprehensive Cancer Network (NCCN) panel recommends LDCT lung screening for two groups of individuals at high risk of developing lung cancer: (I) smokers or former smokers who meet the same criteria (i.e., age, pack-year history, and smoking status) as in the NLST and (II) individuals age 50 years or older (no upper age limit) with a 20 or more pack-year smoking history and with one additional risk factor (24). The AATS advises screening up to age 79 in ever smokers regardless of time since quitting (3). The USPSTF recommendation also increased the upper age limit (80 years) after review of Cancer Intervention and Surveillance Modeling Network (CISNET) modeling studies which still showed benefit among older adults in appropriately selected populations (14).
The NCCN and AATS consider other risk factors (e.g., occupational exposure to carcinogens like asbestos and coal smoke, history of chronic obstructive pulmonary disease) that, in addition to smoking, may synergize to increase risk of lung cancer. Finally, some guidelines acknowledge that absolute risk factor(s) may not be adequate to define eligibility as 2/3 of lung cancer diagnoses would not have met LCS criteria (26). Thus, NCCN suggests risk-based assessments (27) using a risk calculator may help determine a patient’s risk of developing lung cancer and can be used during SDM visits (28).
The role of primary care providers in LCS
The primary care provider is central to implementing a high-quality LCS program. The USPSTF outlined six components of a structured LCS program: (I) identify eligible patients; (II) engage in SDM; (III) refer to certified LCS centers; (IV) follow-up on abnormal findings; (V) manage other health problems during cancer treatment; and (VI) provide tobacco treatment services (29). The literature shows that there are challenges to implementing the first three components.
One study found that only 33% of providers identified eligible patients for LCS (30). A contributing factor may be the lack of awareness or knowledge about the recommendations for LCS. There is variation in the awareness of the guidelines for LCS, ranging from 0% to 89% (30-35). The wide range could be due to the timing of the data collection in relation to when the guidelines were released, confusion due to various guideline eligibility requirements, and the practice setting. For instance, in a qualitative study, none of the primary care providers (N=10) in New Mexico were aware of the new guidelines for LCS (31). They were interviewed from February to September of 2014, which overlapped with the release of the USPSTF recommendations. In contrast, in the study where 89% of the primary care providers (N=36) were aware of the guidelines, they were all from an academic medical center and the data were collected in 2015 after the release of the USPSTF recommendations (35). A larger study with 350 primary care providers found that 86% reported being somewhat or very familiar with the USPSTF guidelines (29). However, many providers do not know the specific eligibility criteria for LCS (32). Only 11 of 36 providers could correctly identify the NLST eligibility criteria for age and smoking history (35). The failure to engage patients in SDM is discussed later in this article.
Although providers may discuss LCS, few are referring patients for screening. An analysis of the National Health Interview Survey showed that in 2015, only 6% of smokers were being screened and chest X-rays were still being ordered for LCS (36). In a study by Duong and colleagues, 27 of the 36 primary care providers reported they discussed LCS and 21 ordered a LDCT for LCS (35). In a study by Lewis and colleagues (37), only 12% of the providers ordered a LDCT. In a more recent survey of providers, 52% ordered LDCT for LCS but only 21% referred patients in the past 12 months (33). A survey study of 350 family medicine providers found that more than half (56%) planned to refer patients for LCS, but only about a quarter (26%) referred patients to a certified LCS center (30). Many providers report assessing for smoking behaviors. However, few are referring patients to formal cessation services (32). More than half (66%) of providers from Texas reported following up on abnormal findings (30). This same study found that nearly 83% of providers manage other health problems during treatment. A recent qualitative study reported that providers had little time for counseling and SDM (32).
What is SDM?
Attention to SDM in healthcare continues to rise. In the U.S. in 1982, the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical Research called for a more equal doctor-patient relationship (38). SDM was advocated as an ethical ideal for patient-professional relationships. The 2009 Institute of Medicine (IOM) Report on Comparative Effectiveness Research defined the purpose of comparative effectiveness research as providing evidence to assist consumers (and by extension patients) to make informed decisions about their care (39).
The landmark IOM Report on “Delivering High-Quality Cancer Care” identified essential components of the healthcare system for cancer patients (40). Two specific recommendations from the IOM address the component about engaging patients in cancer care. Recommendation 1 focuses on information and decision support tools, and Recommendation 2 focuses on improving end of life care through patient-centered approaches, such as clinician training, use of advance care plans, and support and referral to hospice. The goal is to provide patients with high quality information about their care, including use of tools such as decision aids, and training of the care team in providing information.
The Agency for Healthcare Research and Quality (AHRQ) defines SDM as a model of patient-centered care that enables and encourages people to play a role in the medical decisions that affect their health. It operates under two premises: first, consumers armed with good information can and will participate in the medical decision-making process by asking informed questions and expressing personal values and opinions about their conditions and treatment options. Second, clinicians will respect patients’ goals and preferences and use them to guide recommendations and treatments. The aim of SDM is to ensure that patients understand their options and the pros and cons of those options, and patients’ goals and treatment preferences are used to guide decisions (www.ahrq.gov).
In cancer screening, SDM is relevant for a variety of decisions. For LCS, the most recent cancer screening context where SDM is recommended, the primary focus is on the decision to be screened given the potential benefits and known harms. Guidelines indicate an upper age limit at which screening should stop, or when a patient has not smoked within the past 15 years. Guidelines are also clear about the need for annual screening and that LDCT is the only approved screening method. The decision is complex because of the tradeoffs between harms and benefits. In contrast, screening for colorectal cancer includes questions about when to start, when to stop, and which screening modality should be selected (Table 3). Breast cancer screening involves uncertainty about when to start screening and the appropriate screening interval. Finally, prostate cancer screening involves questions about whether or not to be screened, plus when to start, stop, and what interval is appropriate.

Full table
SDM is poorly performed
One of the classic studies in informed decision-making was conducted by Clarence Braddock in the mid to late 1990s (41). Braddock’s group audio-recorded 1,057 encounters among 59 primary care clinicians and 65 general and orthopedic surgeons. The audio-taped discussions were analyzed for seven elements of informed decision-making, reflecting 3 levels of decision complexity: (I) basic decisions (high consensus decision with minimal impact on patient): discuss patient has a role in decision making, and the nature of the decision; (II) intermediate decisions (some uncertainty about options, moderate impact on patient): adds discussion of alternatives and pros/cons of alternatives; and (III) complex decisions (great uncertainty about options, major impact on patient): further adds discussion of uncertainty associated with decision, assesses patient’s understanding, and explores patient’s preferences. Overall, clinicians were doing a very poor job addressing all the elements of informed decision-making. For basic decisions, 17% of encounters met all criteria, while none of intermediate decisions and only one of the complex decisions met all criteria. Elements that were rare included discussing alternatives, pros and cons, the patient’s desired role in decision making, and uncertainties about the choices.
The DECISIONS study, which was conducted by the University of Michigan between November 2006 and May 2007, showed that patients are making uninformed decisions, despite feeling informed, for prostate, colorectal, and breast cancer screening (42). The DECISIONS study surveyed 1,082 adults from the general U.S. population who were 50 years of age or older and had discussed cancer screening with their healthcare provider. Aside from prostate cancer screening, fewer than half of eligible adults reported being asked about their preferences for colorectal (41% for men and 31% for women) and breast cancer (45%) screening. Discussions about the benefits of cancer screening were very common, while discussions of the harms were not. Patients reported that doctors discussed harms of screening for prostate cancer about 30% of the time, colorectal cancer 27% of the time for men and 26% for women, and breast cancer screening 19% of the time. It seems as though there has been little progress since the earlier studies conducted by Braddock’s team.
Strategies for promoting SDM
SDM can be enhanced through the use of patient tools or decision aids, training for clinicians, the use of other members of the clinical team to serve as “decision coaches,” changes in reimbursement for encounters, and redesigning practices to better accommodate opportunities to engage patients in discussions about their healthcare choices. LCS is unique in that CMS reimburses a patient counseling and SDM visit separately from coverage for the actual screening (23). Aside from decision aids, the other strategies have not been explored to any great extent in the context of LCS.
By far, the most widely tested interventions for promoting SDM involve the use of patient decision aids. Patient decision aids are interventions designed to help people make deliberative choices about their healthcare options using the best available evidence. They provide balanced information about options, and help patients construct, clarify, and communicate what is important to them in making values-based choices with their healthcare providers (43,44). The most recent Cochrane systematic review of decision aids for people facing health treatment and screening decisions includes 105 RCTs involving over 31,000 subjects (43). Studies of screening decision aids are common (e.g., prostate cancer, colorectal cancer), but no trials have been conducted on LCS decision aids through April of 2015, the end date for the systematic review.
A model of SDM
Elwyn and colleagues offered a generic, pragmatic model of SDM that can be applied to the LCS decision (45). The “talk” model is meant to support deliberation between clinicians and patients through three steps and lead to informed preferences and shared decisions. We have adapted the model for LCS decisions (Table 4). Any healthcare provider can engage the patient in the SDM process, which is an important consideration for feasibility of the approach in real world clinical settings. Note that CMS has specific criteria for approved non-physicians providing the patient counseling and SDM visit.

Full table
Step 1 is the “Choice Talk” and involves helping the patient understand that LCS is a decision where the patient can choose to be screened or choose not to be screened. It may be done in person, but can also be accomplished before a visit through educational material or a decision aid. Step 2, the “Option Talk”, is a more detailed discussion of LCS, including the possible benefits and harms associated with LDCT screening. Again, a decision aid can be helpful in guiding this discussion and providing information expressed in lay terms about benefits and harms. At this point, techniques such as the “teach-back” method can be used to check the patient’s comprehension of the information and understanding that LCS is a decision. Finally, the “Decision Talk” focuses in exploring the patient’s values and preferences for screening, and making a decision which can include the option of deferring the final decision to a later time.
Tools to support SDM
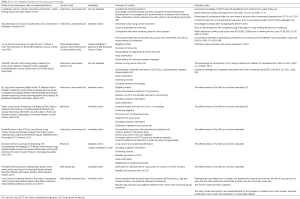
LCS decision aids can support the process of SDM about initiation of LCS. In general, the use of patient decision aids improves the quality of decisions through increased knowledge, more accurate risk perceptions, reduced decisional conflict, improved patient-provider communication, and a better match between what patients’ value and the choices they make (43). Five decision aids are described below; information on these and other decision aids can be found in Table 5. All the decision aids below offer information regarding: lung cancer, LCS process, benefits and harms of LCS, and smoking cessation importance. We will highlight some distinguishing features of each.
Full table
Lung Cancer Screening Decision Aid (LuCaS DA)
LuCaS DA is a decision aid created in response to concerns that most individuals will not take into consideration the potential risks of LCS (54). LuCaS DA includes three main sections: knowledge, empowerment and value clarification. In the knowledge section, screening eligibility and personal risk assessment calculators are included, which follow the NCCN and USPSTF guidelines. The empowerment section aims at improving SDM by developing patients’ deliberative skills. The values clarification section helps patients make decisions consistent with their personal values and preferences. Preliminary results of a study comparing the National Cancer Institute’s (NCI) webpages on LCS with LuCaS DA show that LuCaS DA improves some behavioral outcomes, but is not consistently better than the NCI webpages. Participants with elevated risk of lung cancer (N=50; from Kentucky and SE Florida, USA) were randomized to view the NCI webpages or LuCaS DA webpages and were surveyed after two weeks. LuCaS DA had a high level of acceptability among participants. Increases in knowledge of LDCT and LCS guidelines were shown at the 2-week follow-up (e.g., 33% of DA viewers and 20% of NCI website viewers correctly answered questions concerning mortality reduction). Increases in objective knowledge were not uniformly larger.
shouldiscreen.com
shouldiscreen.com is an online decision aid which provides information about lung cancer and screening, benefits and harms of LCS, comparison with screening for other cancers, and reducing risk of lung cancer presented in texts, graphics, and hyperlinks (48). The site offers an interactive component in which patients calculate pack-years of smoking. Additionally, patients can calculate their personal chance of developing lung cancer in the next 6 years by answering basic demographic and risk factor information. The calculator results also prompt the patient to consider if their personal screening benefit is enough to justify their risk and the reasons for their decision. In 2014, a before-and-after study was conducted to determine the effectiveness of this decision aid on patient-reported outcomes. The results show that knowledge increased and decisional conflict decreased after patients navigated the decision aid. The decision aid had a high level of acceptability, with 97% of the decision aid viewers recognizing it as likely useful for making decisions about LCS (48).
LCS: is it right for me?
“Lung Cancer Screening: Is it right for me?” is a video decision aid designed to influence screening behaviors of high-risk smokers, and facilitate informed decision-making. It consists of four main components: eligibility criteria, an overview of screening, visual display of the magnitude of harms and risks, and values clarification. Additionally, it conveys the important message of smoking cessation and includes a vivid display of pack-years of smoking. An evaluation study of the decision aid has recently been completed and will be reported in 2018.
CHOICE: should I start having yearly screening for lung cancer?
The LCS decision aid CHOICE (Communicating Health Options through Interactive Computer Education) is compliant with the CMS Decision Memo on LCS with LDCT (49). For the evaluation of the decision aid, patients from a diverse population participated in a single-site (academic primary care practice) pre-post pilot study. Participants viewed the 6-minute decision aid video and were instructed to consider their preferences and values. Preliminary results from the pilot study showed the association of viewing the decision aid with greater knowledge about the harms of LCS. The influence of the decision aid on patients’ preparedness for screening and decisional conflict needs further investigation.
Is LCS right for me? A decision aid for people considering LCS with LDCT
The Effective Healthcare Program of the AHRQ developed decision aid tools for patients and healthcare professionals (https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/home.html). The aid includes smoking cessation information with a quit smoking line. Information is presented in text, infographics, and interactive tools. A pack-year calculator is provided in the eligibility criteria section. In the benefits and harms section, factors such as overall mortality benefits, risks from radiation, major complications and overdiagnosis are communicated. This decision tool includes a value clarification section as an interactive survey that helps high-risk smokers make decisions about screening consistent with their preferences. Another component assesses patients’ knowledge of screening and asks their “decision about lung cancer screening” follows the information. Additional guidance on deliberation with a question prompt list, hyperlink for approved LCS centers, and information on insurance coverage are included.
Implementing SDM for LCS
To be effective, interventions to promote SDM must be implemented in routine clinical care. There are many barriers to implementing SDM that cross patient, provider, and practice levels. LCS is becoming a test case for SDM as it is the first preventive healthcare service that requires a visit to discuss harms and benefits of screening for screening to be reimbursed by CMS.
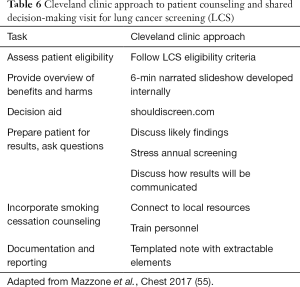
There are several settings where patients and healthcare providers can work together to make informed decisions about LCS. Mazzone and colleagues (16,55) at the Cleveland Clinic developed a centralized model for providing SDM and smoking cessation counseling (Table 6). Their initial strategy was to have primary care providers identify eligible patients and perform SDM in the primary care setting. The approach proved untenable and evolved to a referral model where primary care physicians (PCPs) referred patients to a central LCS program run through a pulmonology clinic. In the LCS program, patients are assessed for eligibility, watch a brief narrated slideshow about LCS, and complete the shouldiscreen.com online patient decision aid to obtain an estimate of their lung cancer risk. Patients and LCS center providers then have a discussion about LCS, make a screening decision, and smokers are connected with local smoking cessation resources. The SDM conversation is delivered by a nurse practitioner, physician assistant, or physician. The program is structured so that interested patients can complete LCS on the same day as the SDM visit. An evaluation of the program showed high acceptability by patients, and gains in knowledge after the SDM visit. Few patients (under 3%) declined screening after the visit, likely due to these patients having accepted a referral for another visit to discuss LCS.
Full table
A second approach is to conduct the patient counseling and SDM visit in the primary care setting. This approach is clearly consistent with the USPSTF recommendation and the reimbursement decision from CMS. Yet, very little is known about how best to implement SDM in busy clinical settings. Previous surveys of PCPs show that many are not familiar with the NLST results, have incomplete information about eligibility for LCS, incorrectly endorse chest radiography as an accepted screening approach, and are somewhat skeptical about the evidence supporting screening (30,31,37,56).
A study of 350 primary care clinicians conducted after the USPSTF recommendations were released assessed current screening practices and implementation needs (30). About 10% of respondents’ practices had a formal LCS program at the time of the survey and less than half (44%) of providers reported engaging patients in SDM prior to referral for LCS. Their implementation needs were as follows: (I) clarity about guidelines/recommendations; (II) information about eligibility criteria; (III) clarity about insurance coverage; (IV) help finding accredited referral centers; (V) SDM tools for patients; (VI) training programs for healthcare providers; and (VII) strategies for integrating screening programs in electronic health records.
An implementation toolkit for LCS in the primary care setting
The Eisenberg Center of the AHRQ released in March 2016, the “Lung Cancer Screening Tools for Patients and Health Care Professionals”. In developing this online toolkit, AHRQ focused on four design goals: (I) providing clinicians with a concise summary of the current evidence and recommendations; (II) providing a way to ensure the patient counseling and SDM visit is consistent with CMS beneficiary eligibility criteria; (III) recognizing that a high-quality patient decision aid was not enough to ensure SDM occurs; and (IV) creating decision support tools in multiple formats and for use in multiple ways to support deliberation between patients and clinicians. The resulting multi-component implementation toolkit can be found here: https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/home.html.
The toolkit contains four components which are readily downloadable for viewing and printing. First is the 2-page “A Summary Guide for Primary Care Clinicians” that provides a summary of the NLST findings including the magnitude of benefits and harms of screening, eligibility criteria for screening, an overview of beneficiary eligibility criteria from CMS, smoking cessation resources, a link to finding accredited imaging centers, and points to discuss with patients. The second tool is the “Lung Cancer Screening: A Clinician’s Checklist” (see Figure 1). The checklist is structured around activities that occur before, during, and after the clinical encounter. The pack-year formula is given with the eligibility criteria. The content of the conversation with the patient about screening harms and benefits, and information about smoking cessation is included. Finally, there is guidance on making a referral and noting the visit in the medical record. The back page of the checklist includes tips on promoting SDM, talking points, and teach-back examples.
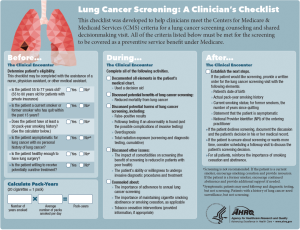
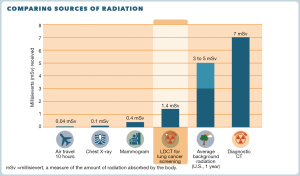
There is also a 4-page patient decision aid, “A Decision Aid for People Considering Lung Cancer Screening”. The aid is meant to be reviewed by the patient before a visit with a healthcare provider to discuss LCS. Features include information about eligibility, messaging about smoking cessation and abstinence, use of icon arrays to describe the magnitude of benefits and harms of LCS, a visual depiction of radiation exposure from LDCT compared to other exposures (see Figure 2), questions to help clarify the patient’s values, questions for the doctor, and information about insurance coverage and other costs. Finally, there is a 2-page abbreviated summary to be used during visits when screening is discussed titled “A Decision-Making Tool for You and Your Health Care Provider”.
The future of SDM for LCS
Here we highlight a few key challenges and opportunities for improving the decisions patients make about LCS. First, implementation of SDM for LCS is key. Successful implementation will go well beyond making high quality decision aids available to healthcare providers and patients. Briefer tools seem appropriate for the LCS context as decisions need to be made between patients and healthcare providers. Involving other members of the clinical team is a promising strategy that may offset some of the burden on already busy physicians. SDM training for other members of the clinical team could build on highly successful models for certifying personnel in tobacco cessation interventions.
Second, there exist huge gaps between what the current evidence about LCS tells us and what patients find important and want to know. These gaps have the potential to undermine SDM if patients feel the issues of importance to them are not reflected in the evidence they review with their healthcare providers. Some of these gaps are depicted in Table 7. The challenge comes from the constraints of large, randomized trials with limited follow-up periods to assess the outcomes of cancer screening interventions. Modeling studies may be the only strategy for addressing these gaps (57), but there remain methodological issues that must be addressed if modeling is to be used to inform individual patient decisions about screening.

Full table
Finally, there is growing interest in tailoring screening to groups most likely to benefit. The advantages of risk tailoring for LCS include: (I) limiting screening to those at highest risk of lung cancer, thereby limiting harms; (II) informing decisions about when to start and stop screening, and how often screening should occur; and (III) providing individualized estimates of benefits and harms to provide a more personalized discussion with the patient. Currently, the role of risk tailoring in supporting SDM in the LDCT LCS context is unclear and worthy of further investigation.
Acknowledgements
Funding: This work was supported in part by grants from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment, project RP160674 from the Cancer Prevention Research Institute of Texas (CPRIT); and through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306-03385).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: All statements in this article, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
References
- World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=lung
- American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society, 2017.
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- Humphrey L, Deffebach M, Pappas M, et al. Screening for Lung Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. Report No.: 13-05188-EF-1. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews.
- Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013;159:411-20. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445-53. [Crossref] [PubMed]
- Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59:355-63. [Crossref] [PubMed]
- Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Agency for Healthcare Research and Quality. Lung Cancer Screening: A Summary Guide for Primary Care Clinicians 2016. Available online: https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/clinician-summary.html
- Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e78S-92S.
- Rocco G, Allen MS, Altorki NK, et al. Clinical statement on the role of the surgeon and surgical issues relating to computed tomography screening programs for lung cancer. Ann Thorac Surg 2013;96:357-60. [Crossref] [PubMed]
- Kazerooni EA, Armstrong MR, Amorosa JK, et al. ACR CT accreditation program and the lung cancer screening program designation. J Am Coll Radiol 2015;12:38-42. [Crossref] [PubMed]
- American Society of Clinical Oncology. What to know: The ACCP and ASCO guideline on lung cancer screening 2012. Available online: http://www.cancer.net/publications-and-resources/what-know-ascos-guidelines/what-know-accp-and-asco-guideline-lung-cancer-screening
- American Lung Association. American lung association provides guidance on lung cancer screening Washington D.C.: American Lung Association; 2012. Available online: http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/
- American Academy of Family Physicians. Evidence Lacking to Support or Oppose Low-dose CT Screening for Lung Cancer, Says AADP 2013. Available online: http://www.aafp.org/news/health-of-the-public/20140113aafplungcarec.html
- Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N) 2015 [February 5, 2015]. Available online: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin 2015;25:185-97. [Crossref] [PubMed]
- Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst 1993;85:457-64. [Crossref] [PubMed]
- Yang P, Wang Y, Wampfler JA, et al. Trends in Subpopulations at High Risk for Lung Cancer. J Thorac Oncol 2016;11:194-202. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. Erratum in: N Engl J Med 2013;369:394. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764. Erratum in: PLoS Med 2015;12:e1001787. [Crossref] [PubMed]
- Richards TB, White MC, Caraballo RS. Lung cancer screening with low-dose computed tomography for primary care providers. Prim Care 2014;41:307-30. [Crossref] [PubMed]
- Volk RJ, Foxhall LE. Readiness of primary care clinicians to implement lung cancer screening programs. Prev Med Rep 2015;2:717-9. [Crossref] [PubMed]
- Hoffman RM, Sussman AL, Getrich CM, et al. Attitudes and Beliefs of Primary Care Providers in New Mexico About Lung Cancer Screening Using Low-Dose Computed Tomography. Prev Chronic Dis 2015;12:E108. [Crossref] [PubMed]
- Kanodra NM, Pope C, Halbert CH, et al. Primary Care Provider and Patient Perspectives on Lung Cancer Screening. A Qualitative Study. Ann Am Thorac Soc 2016;13:1977-82. [Crossref] [PubMed]
- Raz DJ, Wu GX, Consunji M, et al. Perceptions and Utilization of Lung Cancer Screening Among Primary Care Physicians. J Thorac Oncol 2016;11:1856-62. [Crossref] [PubMed]
- Raz DJ, Wu GX, Consunji M, et al. The Effect of Primary Care Physician Knowledge of Lung Cancer Screening Guidelines on Perceptions and Utilization of Low-Dose Computed Tomography. Clin Lung Cancer 2018;19:51-7. [Crossref] [PubMed]
- Duong DK, Shariff-Marco S, Cheng I, et al. Patient and primary care provider attitudes and adherence towards lung cancer screening at an academic medical center. Prev Med Rep 2017;6:17-22. [Crossref] [PubMed]
- Huo J, Shen C, Volk RJ, et al. Use of CT and Chest Radiography for Lung Cancer Screening Before and After Publication of Screening Guidelines: Intended and Unintended Uptake. JAMA Intern Med 2017;177:439-41. [Crossref] [PubMed]
- Lewis JA, Petty WJ, Tooze JA, et al. Low-Dose CT Lung Cancer Screening Practices and Attitudes among Primary Care Providers at an Academic Medical Center. Cancer Epidemiol Biomarkers Prev 2015;24:664-70. [Crossref] [PubMed]
- Making Health Care Decisions Volume One: Report 1982 [cited 2018 Feb. 7]. Available online: http://hdl.handle.net/10822/559354
- Ratner R EJ, Wolman D, Greenfield S, et al. editors. Institute of Medicine. Initial national priorities for comparative effectiveness research. Washington, DC: National Academies Press, 2009:200.
- IOM (Institute of Medicine). Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: The National Academies Press, 2013.
- Braddock CH 3rd, Edwards KA, Hasenberg NM, et al. Informed decision making in outpatient practice: time to get back to basics. JAMA 1999;282:2313-20. [Crossref] [PubMed]
- Hoffman RM, Lewis CL, Pignone MP, et al. Decision-making processes for breast, colorectal, and prostate cancer screening: the DECISIONS survey. Med Decis Making 2010;30:53S-64S. [Crossref] [PubMed]
- Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [PubMed]
- Volk RJ, Llewellyn-Thomas H, Stacey D, et al. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak 2013;13 Suppl 2:S1. [PubMed]
- Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med 2012;27:1361-7. [Crossref] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Implementation of Lung Cancer Screening: Proceedings of a Workshop. Washington, DC: The National Academies Press, 2017. Available online: https://doi.org/ [Crossref]
- Byrne MM, Brinker K, Eckman C, et al. Improving knowledge about lung cancer screening: effect of a decision aid vs. NCI website. 38th Annual North American Meeting of the Society for Medical Decision Making; October 25, 2016.
- Lau YK, Caverly TJ, Cao P, et al. Evaluation of a Personalized, Web-Based Decision Aid for Lung Cancer Screening. Am J Prev Med 2015;49:e125-9. [Crossref] [PubMed]
- Reuland DS, Cubillos L, Brenner AT, et al. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med Inform Decis Mak 2018;18:5. [Crossref] [PubMed]
- Reuland DS, Brenner A, Cubillos L, et al. Effect of a lung cancer screening decision aid on knowledge of screening harms and screening preference in primary care patients. 38th Annual North American Meeting of the Society for Medical Decision Making; Sunday, October 23, 2016.
- Agency for Healthcare Research and Quality. Is Lung Cancer Screening Right for Me? A Decision Aid for People Considering Lung Cancer Screening with Low-Dose Computed Tomography. Agency for Healthcare Research and Quality, 2016.
- Samson P, et al. Yearly Lung Cancer Screening: Is It Right for Me? Apr. 2016. Available online: https://siteman.wustl.edu/wp-content/uploads/2016/04/Lung-Cancer-Screening-20160427_rev.pdf
- Bach PB, Gould MK. When the average applies to no one: Personalized decision making about potential benefits of lung cancer screening. Ann Intern Med 2012;157:571-3. [Crossref] [PubMed]
- Studts J, Brinker K, Tannenbaum S, et al. LuCaS DA: A lung cancer screening decision aid to improve screening decisions. J Thorac Oncol 2017;12:S577. [Crossref]
- Mazzone PJ, Tenenbaum A, Seeley M, et al. Impact of a Lung Cancer Screening Counseling and Shared Decision-Making Visit. Chest 2017;151:572-8. [Crossref] [PubMed]
- Ersek JL, Eberth JM, McDonnell KK, et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer 2016;122:2324-31. [Crossref] [PubMed]
- de K·oning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:311-20.


