Extrapulmonary neoplasms in lung cancer screening
Introduction
The National Lung Screening Trial (NLST) showed that low-dose computed tomography (LDCT) screening reduced lung cancer specific mortality rate in high-risk patients by 20% compared with chest radiography, with a 6.7% reduction in all-cause mortality (1). Besides early detection of lung cancer, LDCT screening provides an opportunity for detection and early treatment of significant and potentially significant incidental findings (IF), defined as actionable abnormalities seen on LDCTs that are outside the screening purview of the study, including extrapulmonary neoplasms. Unlike other screening tests such as mammography that focus on imaging a single organ, lung cancer screening (LCS) cross-sectional images are acquired from the lower neck to the upper abdomen including multiple organs, allowing detection of a significant number of IF.
IFs on lung LCS
Extrapulmonary findings on LCS are very common. A study by Nguyen and colleagues that reviewed prospectively acquired data on 17,309 NLST participants showed that extrapulmonary findings were noted in 58.7% of the CT-screened participants, and 19.6% had findings coded as potentially significant (2). The impact of IFs was also evaluated by Kucharczyk and colleagues (3) who evaluated 4,073 LDCTs from LCS participants in Canada and found similar results: a total of 880 IFs described in 782 study participants from 807 different scans (19%). Of these, 689 were considered non-cardiovascular (78%). The majority of the IFs were identified at baseline imaging (80%). There was a correlation between IF prevalence and age and pack-years of smoking; pack-years were significantly higher in the group with IFs (P=0.04). The reported frequency of clinically significant extrapulmonary IFs (other than coronary artery calcification) in other screening studies ranges from 7% to 27% (4-7); this variation is at least in part due to inconsistent definition of what constitutes an actionable IF and different methodology for recording IFs in the different studies.
LDCT lacks the accuracy required for making a definitive diagnosis for most potentially significant IFs outside of the lung parenchyma. As a result, further diagnostic workup of the IFs is frequently recommended by the radiologist. The clinical relevance of these IFs is still controversial. Considering the fact that 22.3% of the certified deaths (416 of 1,865) in the CT arm of the NLST trial were due to extrapulmonary malignancies, compared to 22.9% of deaths from lung cancer (427 of 1,865) (1), it is possible that early diagnosis and treatment of clinically significant IF may further decrease morbidity and mortality in screening participants. However, the potential for harm includes anxiety, cost and morbidity related to additional workup of findings that may prove to be of no clinical relevance. These potential consequences of detection of IF should be discussed with patients during the shared decision-making visit. In addition, the cost-effectiveness of LCS could be negatively impacted by the detection of a large number of IF, particularly if subsequent workup is not performed appropriately, so standardization of management is critical.
Prevalence of extrapulmonary malignancy
The prevalence of extrapulmonary malignancies presenting as IF in LDCT LCS participants published in the literature varies from 0–1.6% (1,3,4,6-10). This range can be explained by differences in the number of participants enrolled, variations in CT scanning protocol, including scanning range, differences in duration of surveillance and potential inclusion of additional imaging modalities, particularly PET/CT, in national and international LDCT screening studies. This variability also results in a significant variation in the type of reported extrapulmonary neoplasms in each study.
According to Nguyen et al. (2), the prevalence of potentially significant abnormalities in CT-screened NLST participants was highest for cardiovascular findings (8.5%), followed by renal (2.4%), hepatobiliary (2.1%), adrenal (1.2%), and thyroid (0.6%) findings. Sixty-seven of 17,309 participants (0.39%) had primary extrathoracic cancers diagnosed during screening. The prevalence of cancers among screened participants was 0.26% (45 cases) for kidney, 0.08% (14 cases) for thyroid, and 0.05% (8 cases) for liver cancers. There were no adrenal malignancies.
In another study, Rampinelli et al. (7) identified 27 extrapulmonary malignancies among 5,201 participants (0.5%) undergoing 5 years of LDCT screening in the COSMOS (Continuous Observation of Smoking Subjects) study. Eight malignancies were diagnosed in the 1st year of screening, 9 in the 2nd year, 4 in the 3rd year, 2 in the 4th year, and 4 in the 5th year. The most commonly diagnosed malignancies (44%) were renal cell carcinoma (7) and lymphoma (5). Other tumor types included thyroid cancer (3), thymoma (2), pancreatic neoplasm (2), schwannoma (1), hepatocellular carcinoma (1), gastrointestinal stromal tumor (1), prostate cancer (1), breast cancer (1), adrenal gland neoplasm (1) and ovarian cancer (1). Importantly, the extrapulmonary neoplasms were overlooked by the initial readers in five participants, but could be retrospectively detected (7). Although lymphoma was the second most common malignancy in this study, most incidentally detected enlarged mediastinal nodes in chest CT have proven to be benign, most commonly reactive to an infectious process, pulmonary edema, and diffuse lung disease, such as fibrosis or sarcoidosis. Less frequently, incidentally detected enlarged mediastinal nodes represent metastatic disease, small cell lung cancer or lymphoma. In a study that investigated incidental mediastinal lymphadenopathy with positron emission tomography, endoscopic ultrasound (EUS)- or endobronchial ultrasound-guided biopsies, and had clinical follow-up in 83 patients, 66% of the nodes were reactive and 22% represented sarcoidosis; only one case of metastatic disease was seen in a patient with known breast cancer (11).
Historically, the frequency of incidental breast findings on diagnostic chest CT has been reported as 1.1% and the frequency of malignant findings as 0.3–0.4%, i.e., 1 out of 250 women undergoing chest CT will show a malignant incidental breast lesion (12,13). Concordantly, in the study by Kucharczyk and colleagues (3), of the 7 incidentally detected malignancies (0.8%), 4 were breast cancers and the remainder were 2 plasmacytomas of the rib and 1 thyroid cancer.
In the Early Lung Cancer Action Project (ELCAP), Henschke and colleagues (14) evaluated the frequency and temporal evolution of mediastinal masses found in screening participants. Of the 9,263 study participants, 71 had a mediastinal mass at baseline screening (prevalence of 0.77%). Of the 71 masses, 41 were thymic, 16 were thyroidal, 2 were esophageal cancers, 6 were tracheal-esophageal diverticula, and 6 were other masses, including cystic lesions. Among the 11,126 annual repeat screenings, only one new mediastinal mass was identified (incidence of 0.01%). This suggests a long average duration for mediastinal masses in asymptomatic individuals. Among the 41 thymic masses, five were larger than 3.0 cm in diameter, and all five were resected; of these 5, 1 was a thymic carcinoma and 4 were noninvasive thymomas. Of the remaining 36 thymic masses, 25 were evaluated at follow-up CT 1 year later: 5 had increased in diameter, 2 had decreased, and 18 remained unchanged (14).
In contrast to the previous studies, van de Wiel et al. (6) reported that the incidental detection of extrapulmonary malignancies in the Dutch-Belgian lung cancer screening trial (NELSON) was negligible. Among the 1,929 participants, 129 (7%) had clinically significant IFs of which only one was a malignancy. Furthermore, detection of this metastatic pancreatic malignancy was not associated with clinical benefit since no curative treatment was possible.
Proposed guidelines for management of extrapulmonary IFs in LCS
Currently, there are no specific recommendations to determine which IFs should be considered relevant in LCS, although future editions of American College of Radiology (ACR) Lung-RADS (Lung CT Screening Reporting and Data System), used to standardize LDCT screening interpretation, reporting and recommendations for management of identified lesions, may address this issue. In this regard, Lung-RADS version 1.0 classification leaves the relevance of detected IFs to the discretion of the reader and simply provides a category S modifier for screening participants who have a clinically significant or potentially clinically significant non-lung cancer finding.
While IFs should be evaluated on a case-by-case basis, there are general guidelines available for the management of incidentally detected lesions in the thyroid, liver, kidney, pancreas and adrenal glands (15-18). These guidelines are important to ensure patients receive appropriate medical care and to minimize unnecessary diagnostic workup that can decrease the cost-effectiveness of LCS and add to the overall health care economic burden.
Thyroid gland
The incidental thyroid nodule is one of the most common IFs on imaging studies that include the neck. A white paper on the management of IFs in the thyroid gland recommends evaluation with thyroid ultrasound, and possibly fine needle aspiration (FNA) depending on ultrasound findings, in patients with nodules >1.5 cm (for patients age ≥35 years, which includes LCS participants) and/or nodules with findings suspicious for malignancy including microcalcifications, mixed solid/cystic attenuation, local invasion as well as associated lymphadenopathy and/or nodules that are fluorodeoxyglucose (FDG) avid on PET/CT imaging (15).
Mediastinum
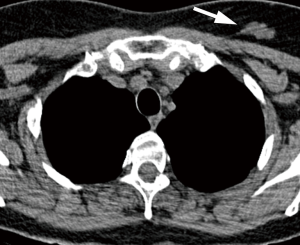
There is little literature regarding the importance of incidentally detected mediastinal findings. According to available data, most mediastinal masses found in the context of CT screening for lung cancer in asymptomatic people can be approached in a “conservative” manner; this includes thymic masses smaller than 3 cm in diameter, as most of these remain unchanged or even decrease in size (14). In these cases, a short-term follow-up LDCT or PET/CT imaging may be considered to exclude active malignancy, followed by continued follow-up at annual LDCT screening if stable (Figure 1). Mediastinal lesions ≥3 cm may require further investigation with contrast-enhanced chest CT or MRI, except those with a typical benign cystic appearance.
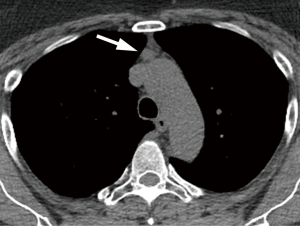
Esophageal cancer is not a common IF, but the presence of focal esophageal wall thickening or esophageal mass should prompt further investigation with endoscopic evaluation (Figure 2).
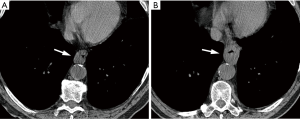
Lymphadenopathy
According to ACR recommendations for the management of incidentally detected mediastinal lymph nodes, depending on size, texture, and distribution of the nodes, options include reporting as probably benign needing no follow-up, or indeterminate and recommending follow-up with CT, PET/CT and/or biopsy (19). Incidentally detected mediastinal lymph nodes <15 mm (in short axis) in patients with no other findings do not require further evaluation (19). Mildly enlarged mediastinal and hilar nodes are also frequently seen in association with pulmonary findings suggestive of pulmonary edema, fibrosis, infection, collagen vascular disease, or sarcoidosis, and generally require no additional workup. Consideration for short-term follow-up CT or other diagnostic procedures is made in cases of isolated, significantly enlarged lymph nodes (Figure 3) (20). The lack of benign lymph node features, such as smooth and well-defined borders, uniform attenuation, and central fatty hilum, or the loss of these features since the previous examination, should raise suspicion of a clinically significant condition and require further investigation (19).
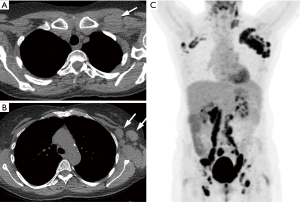
Breast
Breast incidentalomas without the pathognomonic appearance of fibroadenomas (coarse calcifications) may require further work-up with mammography and/or dedicated ultrasound (Figure 4). If available, correlation with mammography or previous chest CT examinations should be obtained. If the lesion is confirmed to be new or shows increase in size, it should be regarded as a finding suspicious for breast malignancy (21).
Upper abdomen
Recently the ACR updated recommendations for the management of abdominal IFs detected on diagnostic imaging studies (16-18). These recommendations can, to a large part, be adapted to manage incidentally detected abdominal findings in the LCS population.
Liver lesions <1 cm in low-risk patients and lesions with benign features (sharply marginated with homogeneous low attenuation 0–20 HU) require no additional workup. For liver lesions ≥1 cm without distinctly benign features and liver lesions in high risk patients (with known primary malignancy with a propensity to metastasize to the liver, cirrhosis, and/or other hepatic risk factors), liver MRI is recommended (16).
Renal lesions that are sharply marginated with homogeneous low attenuation (0–20 HU) require no further evaluation. Renal lesions with attenuation 20–69 HU or those with thick or irregular wall, mural nodule, calcification or septa, should be further evaluated with MRI or CT without and with intravenous contrast (17).
Adrenal gland lesions that have macroscopic fat or attenuation ≤10 HU or lesions that are stable in size for 12 months require no further evaluation. In addition, an incidental adrenal lesion that is <1 cm in short axis usually does not need be further investigated. Incidental adrenal lesions measuring 1–2 cm with no prior imaging in patients with no cancer history are still probably benign; follow-up adrenal protocol CT in 12 months has been recommended in the general population. However, in the LCS population undergoing annual LDCT, evaluation for lesion stability at the 12-month LDCT follow-up may be sufficient (Figure 5). Adrenal masses >2 cm (or ≥1 cm in patients with cancer history) and <4 cm should be evaluated with adrenal protocol CT. Adrenal masses ≥4 cm without benign features in patients without history of prior cancer will require consideration for resection (without biopsy) to treat possible primary adrenal cortical carcinoma. Correlation with clinical signs or symptoms (hypertension, Cushing’s features) may suggest a biochemically active neoplasm. For adrenal masses ≥4 cm in patients with prior history of cancer, biopsy or PET/CT is recommended to exclude metastatic disease (18). Both benign and malignant adrenal masses may enlarge over time, and there is not a known growth-rate threshold to differentiate benign from malignant adrenal masses (22). New or enlarging adrenal lesions on annual LDCT should be considered for adrenal CT protocol evaluation or resection (no cancer history); or biopsy or PET/CT evaluation (if prior cancer history) (18).
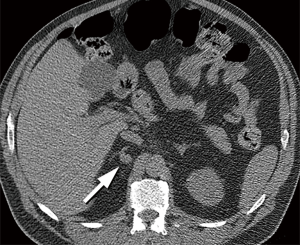
Incidentally detected solid pancreatic lesions are almost always consistent with pancreatic adenocarcinoma or neuroendocrine tumor and should be properly evaluated with pancreas protocol CT and/or MRI; they usually require an aggressive approach with biopsy and/or surgery, except in elderly patients with small neuroendocrine tumors (Figure 6). Incidentally detected pancreatic cysts are presumed to be mucinous unless proven otherwise. Current recommendation for pancreatic cysts <1.5 cm is evaluation with contrast-enhanced MRI or pancreas protocol CT annually for patients <65 years old and every 2 years for patients ≥65 years old for 5 years, and then annually for an additional 5 years, up to the age of 80 years (23). Cysts that demonstrate interval growth (≥20% increase in longest axis diameter) may require more frequent imaging surveillance or EUS/FNA. The evaluation of larger lesions will depend on lesion size, communication with the main pancreatic duct, presence of suspicious features, such as thickened/enhancing wall or solid component, as well as interval growth. Evaluation may require CT or MRI with magnetic resonance cholangiopancreatography (MRCP), and possible EUS/FNA and surgical consultation (Figure 7) (23).
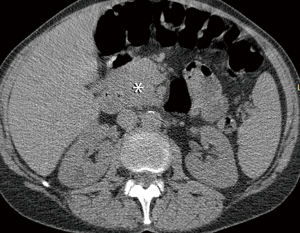
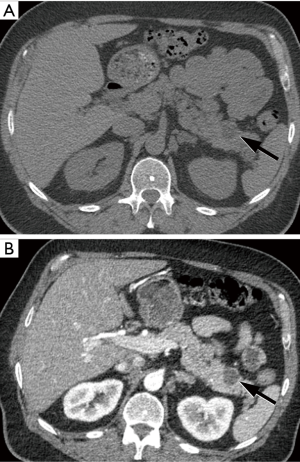
In summary, a considerable number of IF can be identified in LCS participants, including extrapulmonary malignancies in a small percentage of cases. Early detection and treatment of these neoplasms may potentially decrease mortality and morbidity in lung screening participants, but further studies are needed to evaluate the effect of treatment intervention on patient outcome. The possible consequences related to IFs should be discussed with patients during shared decision-making visits. The use of standardized guidelines for reporting and management of IFs is warranted to avoid unnecessary workup and cost while maintaining optimal clinical care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Nguyen XV, Davies L, Eastwood JD, et al. Extrapulmonary Findings and Malignancies in Participants Screened With Chest CT in the National Lung Screening Trial. J Am Coll Radiol 2017;14:324-30. [Crossref] [PubMed]
- Kucharczyk MJ, Menezes RJ, McGregor A, et al. Assessing the impact of incidental findings in a lung cancer screening study by using low-dose computed tomography. Can Assoc Radiol J 2011;62:141-5. [Crossref] [PubMed]
- MacRedmond R, Logan PM, Lee M, et al. Screening for lung cancer using low dose CT scanning. Thorax 2004;59:237-41. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508-13. [Crossref] [PubMed]
- van de Wiel JC, Wang Y, et al. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol 2007;17:1474-82. [Crossref] [PubMed]
- Rampinelli C, Preda L, Maniglio M, et al. Extrapulmonary malignancies detected at lung cancer screening. Radiology 2011;261:293-9. [Crossref] [PubMed]
- Chiles C, Paul NS. Beyond lung cancer: a strategic approach to interpreting screening computed tomography scans on the basis of mortality data from the National Lung Screening Trial. J Thorac Imaging 2013;28:347-54. [Crossref] [PubMed]
- Priola AM, Priola SM, Giaj-Levra M, et al. Clinical implications and added costs of incidental findings in an early detection study of lung cancer by using low-dose spiral computed tomography. Clin Lung Cancer 2013;14:139-48. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003;226:756-61. [Crossref] [PubMed]
- Stigt JA, Boers JE, Oostdijk AH, et al. Mediastinal incidentalomas. J Thorac Oncol 2011;6:1345-9. [Crossref] [PubMed]
- Surov A, Fiedler E, Wienke A, et al. Intramammary incidental findings on staging computer tomography. Eur J Radiol 2012;81:2174-8. [Crossref] [PubMed]
- Monzawa S, Washio T, Yasuoka R, et al. Incidental detection of clinically unexpected breast lesions by computed tomography. Acta Radiol 2013;54:374-9. [Crossref] [PubMed]
- Henschke CI, Lee IJ, Wu N, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586-90. [Crossref] [PubMed]
- Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol 2015;12:143-50. [Crossref] [PubMed]
- Gore RM, Pickhardt PJ, Mortele KJ, et al. Management of Incidental Liver Lesions on CT: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14:1429-37. [Crossref] [PubMed]
- Herts BR, Silverman SG, Hindman NM, et al. Management of the Incidental Renal Mass on CT: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2018;15:264-73. [Crossref] [PubMed]
- Mayo-Smith WW, Song JH, Boland GL, et al. Management of Incidental Adrenal Masses: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14:1038-44. [Crossref] [PubMed]
- Munden RF, Chiles C, MacMahon H, et al. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2018. In press.
- Tsai EB, Chiles C, Carter BW, et al. Incidental Findings on Lung Cancer Screening: Significance and Management. Semin Ultrasound CT MR 2018;39:273-81. [Crossref] [PubMed]
- Gossner J. Intramammary Findings on CT of the Chest - a Review of Normal Anatomy and Possible Findings. Pol J Radiol 2016;81:415-21. [Crossref] [PubMed]
- Pantalone KM, Gopan T, Remer EM, et al. Change in adrenal mass size as a predictor of a malignant tumor. Endocr Pract 2010;16:577-87. [Crossref] [PubMed]
- Megibow AJ, Baker ME, Morgan DE, et al. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14:911-23. [Crossref] [PubMed]


