How old is “too old” for translational research?
Introduction
It is first necessary to define what we mean by an “old” person. The age threshold depends on the country, being generally 65 years in English-speaking countries, and 75 years in Europe. The most commonly used threshold is 70 years.
Whatever the chosen age definition, most cases of lung cancer occur during the latter part of life, 50% of cases over 65 years, 30% after 75 years (1,2). It is therefore important to care for these patients in the best possible conditions.
Geriatric indexes (3-5) have been developed in recent years to evaluate these patients. At the same time, questions have arisen as to the use of targeted therapies in this population. Even if chemotherapy has proven effective (6), it is reserved for patients in good general condition and does not take into account all the dimensions of these patients’ functional changes (metabolic, pharmacological) or their comorbidities (7).
This raises the question of whether we can apply to this population the same translational approach as that used for younger patients (personalized chemotherapy, targeted therapy).
Biological modifications
A recent review of the literature (8) showed that aging has nine major biological effects: genomic instability, altered telomeres, epigenetic changes (affecting histone deacetylase inhibitors), leading to chromosomal instability, loss of hemostasis, growth hormone (GH) dysregulation, mitochondrial dysfunction, cellular senescence, impaired cell-cell communication, and impaired stem cell activity in blood.
The most striking modifications affect the anterior pituitary, with a potential impact on insulin-like growth factor 1 (IGF-1). This pathway (Figure 1), which is the last to be affected by aging, impacts multiple activation pathways (PTEN, PI3K, FOXO, mTOR and ROS) which may also be involved in lung cancer.
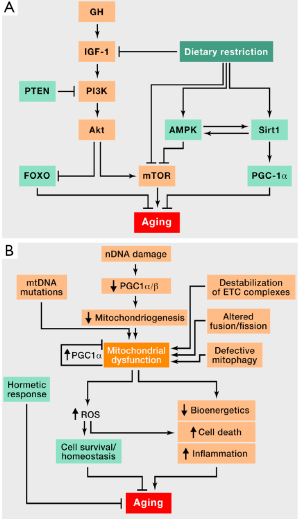
One must therefore take into account not only the physiological changes of aging but also factors also involved in the development of lung cancer. Further research is needed to identify links between biological aging and pathways involved in pulmonary carcinogenesis.
Targeted therapeutics in NSCLC
One of the main issues facing these patients is which treatment to choose first. Tyrosine kinase inhibitors (TKIs) have a recognized role, initially being assimilated to the “Lazarus syndrome” (9,10). The first trials in elderly patients involved gefitinib (Table 1). Gefitinib does not have more major adverse effects in this population than in younger patients.
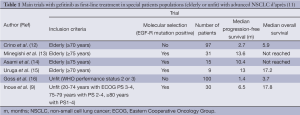
Full table
Trials were subsequently conducted with erlotinib (17-20), including one devoted specifically to patients over 80 years of age (17). This trial, conducted in China, involved 203 patients, of whom 75 (32%) received first-line TKI therapy, while 46% received supportive care alone, and the remainder received chemotherapy. Two recents French trials have compared erlotinib first versus chemotherapy and the opposite sequence in fit (21) and unfit patients (22). Whatever could be the sequence, results were similar between the two groups.
However, epidermal growth factor receptor (EGF-R) mutated patients treated with TKIs were most benefited in terms of overall survival compared to EGF-R wild type patients. Survival was also better in EGF-R wild-type patients treated with erlotinib compared to those treated with chemotherapy.
There are no specific trials of angiogenesis inhibitors in elderly lung cancer patients.
Antiangiogenic agents are also widely used for lung cancer treatments.
In the ECOG 4599 trial (23), comparing carboplatin-paclitaxel to carboplatin-paclitaxel-bevacizumab. Bevacizumab did not improve survival in the subgroup of patients aged 70 years or more (median 74 years), although there was a trend towards a better response rate and longer progression-free survival in the bevacizumab group. Toxicity, and especially hematologic adverse effects, was higher in the bevacizumab arm. In the AVAIL study (24) of cisplatin-gemcitabine with or without bevacizumab, progression-free survival was significantly better with bevacizumab and was similar in the older and younger subgroups, without specific toxicity in the older group; however, the median age of patients over 65 was only 68 years. In the ARIES prospective cohort study (25) evaluating the use of bevacizumab in combination with first-line chemotherapy, progression free survival (PFS) was respectively 6.6 and 6.7 months in patients <70 years (n=1,320) and ≥70 years (n=647), and overall survival was respectively 14.2 and 12.2 months, i.e., largely inferior in patients ≥70 years. There was no excess toxicity in these latter patients.
The role of bevacizumab combined with platinum-based chemotherapy in patients ≥70 years of age needs to be prospectively evaluated in a phase III trial specifically dedicated to elderly patients.
Conclusions
There is thus no reason why elderly patients should not receive targeted therapies, provided that their general condition and comorbidities are taken into account. In the future, it may be possible to identify the patient subgroups most likely to benefit from the treatment options like first-line targeted therapy, or a combination of targeted therapy with chemotherapeutic agents with proven activity in elderly patients. This may be better accomplished by biological profiling of the disease, which may be easier performed in liquid biopsies, like cfDNA or CTCs, as tissue acquisition through a rebiopsy is often difficult in “old” patients. Indeed, as Tsao et al. (26) showed in the BATTLE trial, elderly patients should not have to forego biological analyses. Screening for known genetic abnormalities (27,28) in this high-incidence population will lead to improved survival (28), in combination with clinical tools.
Acknowledgements
Presented at the 10th Biannual GECP Lung Cancer Meeting, 24th November 2013, Barcelone.
Disclosure: The authors declare no conflict of interest.
References
- Balducci L. Lung cancer and aging, Educational book, ASCO 2005:587-91.
- NCI. National Cancer Institute, Surveillance Epidemiology and End Results data. 2013; Available online: http://seer.cancer.gov/, October 2013.
- Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist 2000;5:224-37. [PubMed]
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-31. [PubMed]
- Overcash JA, Beckstead J, Extermann M, et al. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol 2005;54:129-36. [PubMed]
- Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079-88. [PubMed]
- Tam TC, Ho JC, Wong MK, et al. Treatment outcomes in elderly with advanced-stage non-small cell lung cancer. Lung 2013;191:645-54. [PubMed]
- López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013;153:1194-217. [PubMed]
- Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-400. [PubMed]
- Langer CJ. The “lazarus response” in treatment-naive, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol 2009;27:1350-4. [PubMed]
- Gridelli C, De Marinis F, Di Maio M, et al. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating Epidermal Growth Factor Receptor mutation: implications for clinical practice and open issues. Lung Cancer 2011;72:3-8. [PubMed]
- Crinò L, Cappuzzo F, Zatloukal P, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II study. J Clin Oncol 2008;26:4253-60. [PubMed]
- Minegishi Y, Maemondo M, Okinaga S, et al. First-line gefitinib therapy for elder advanced non-small cell lung cancer patients with epidermal growth factor receptor mutations: Multicenter phase II trial (NEJ 003 study). J Clin Oncol 2010;28:abstr 7561.
- Asami K, Koizumi T, Amejima S, et al. First-line gefitinib for elderly patients harboring EGFR mutations. J Clin Oncol 2010;28:abstr e18094.
- Uruga H, Kishi K, Fujii T, et al. Efficacy of gefitinib for elderly patients with advanced non-small cell lung cancer harboring epidermal growth factor receptor gene mutations: a retrospective analysis. Intern Med 2010;49:103-7. [PubMed]
- Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol 2009;27:2253-60. [PubMed]
- Chen KY, Chen JH, Shih JY, et al. Octogenarians with advanced non-small cell lung cancer: treatment modalities, survival, and prognostic factors. J Thorac Oncol 2010;5:82-9. [PubMed]
- Ebi N, Semba H, Tokunaga SJ, et al. A phase II trial of gefitinib monotherapy in chemotherapy-naive patients of 75 years or older with advanced non-small cell lung cancer. J Thorac Oncol 2008;3:1166-71. [PubMed]
- Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol 2012;7:1417-22. [PubMed]
- Wu CH, Fan WC, Chen YM, et al. Second-line therapy for elderly patients with non-small cell lung cancer who failed previous chemotherapy is as effective as for younger patients. J Thorac Oncol 2010;5:376-79. [PubMed]
- LeCaer H, Greillier L, Corre R, et al. A multicenter phase II randomized trial of gemcitabine followed by erlotinib at progression, versus the reverse sequence, in vulnerable elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0505 study). Lung Cancer 2012;77:97-103. [PubMed]
- LeCaer H, Barlesi F, Corre R, et al. A multicentre phase II randomised trial of weekly docetaxel/gemcitabine followed by erlotinib on progression, vs the reverse sequence, in elderly patients with advanced non small-cell lung cancer selected with a comprehensive geriatric assessment (the GFPC 0504 study). Br J Cancer 2011;105:1123-30. [PubMed]
- Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 2008;26:60-5. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [PubMed]
- Wozniak AJ, Garst J, Jahanzeb M, et al. Clinical outcomes (CO) for special populations of patients (pts) with advanced non-small cell lung cancer (NSCLC): results from ARIES, a bevacizumab (BV) observational cohort study (OCS). J Clin Oncol 2012;28:abstr 7618.
- Tsao AS, Liu S, Lee JJ, et al. Clinical outcomes and biomarker profiles of elderly pretreated NSCLC patients from the BATTLE trial. J Thorac Oncol 2012;7:1645-52. [PubMed]
- Rosell R, Santarpia M, Moran T, et al. Age-related genetic abnormalities: the Achilles’ heel for customizing therapy in elderly lung cancer patients. Personal Med 2007;4:59-72.
- Rosell R. Pharmacogenomics comes of age in selecting patients for lung cancer treatment. American Society of Clinical Oncology, 42nd. Annual Meeting, Educational Book, 2006:425-30.


