
Stereotactic body radiotherapy as salvage treatment for recurrence of non-small cell lung cancer after prior surgery or radiotherapy
Introduction
For patients with recurrent non-small cell lung cancer (NSCLC), treatment options are often limited. Resection may not be feasible due to the location or extent of recurrence and/or patient lung and functional status. Radiotherapy may be appropriate for select patients with recurrent NSCLC, though potential benefits of radiotherapy must be weighed against risks. The use of novel technologies has been used to minimize toxicity risks.
Stereotactic body radiation therapy (SBRT), also called stereotactic ablative body radiotherapy (SABR), uses advanced imaging and localizing techniques to optimize radiotherapy targeting accuracy which facilitates the delivery of hypofractionated, ablative doses of radiation (1). SBRT is effective by virtue of delivering therapeutic radiation doses that are associated with relatively high tumor control probability, while minimizing the volume of normal tissue exposed to these therapeutic doses. Thoracic SBRT is minimally invasive, and is therefore well-suited for salvage therapy after prior thoracic radiation therapy or prior thoracic resection. A recent American Society of Radiation Oncology consensus statement discussed the role of SBRT for salvage therapy after 3 scenarios: prior conventionally fractionated radiation, prior SBRT and prior sublobar resection (2). In all 3 scenarios, the quality of evidence was considered low and it was recommended that treatment considerations be individualized to each patient. For patients previously treated with conventionally fractionated radiation therapy, SBRT may allow for a different biologic mechanism of radiation injury (3), thus potentially overcoming apparent radiation resistance. Re-irradiation is associated with extra toxicity risks, which need to be weighed against the benefit of salvage radiation therapy for these patients (4). For patients who have undergone prior resection, SBRT may allow for relatively greater lung sparing (compared to a salvage resection which would likely require a lobectomy or pneumonectomy after prior sublobar resection). However, these patients are also subject to potential radiotherapy-related toxicity from salvage therapy, just as for other patients with no prior therapy (4-8). Notably, a different scenario is consideration of resection for local recurrence after SBRT for NSCLC, a scenario we will not review here, but for which data have been published (9-15).
Here, we review the outcomes of patients undergoing salvage SBRT for pulmonary recurrences after prior radiotherapy or prior resection for NSCLC.
SBRT for thoracic re-irradiation
When considering re-irradiation for recurrent NSCLC, important factors include the prior dose and dose-fractionation, duration of time between course of radiation therapy, location of recurrences/radiation target(s), patient performance status and intent of therapy (curative vs. palliative) (4). Incorporating these factors in a meaningful way to optimize salvage therapy for patients (i.e., best dose-fractionation schedule) with respect to normal tissue complication risks and tumor control probability would be ideal, although the published literature to date does not provide enough data to help us select the best treatment for patients.
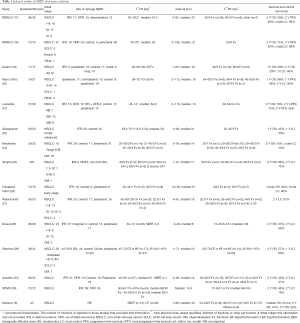
Table summarizes published studies (all retrospective) of patients undergoing salvage SBRT after prior radiotherapy for thoracic cancer (with studies selected that included all or mostly patients with recurrent NSCLC) (16-30) (Table 1). Following salvage SBRT, after prior external beam radiation (SBRT or conventionally fractionated), the 2-year OS ranged from 37% to 79% (median 47%) in 11 studies reviewed here, while the 2-year LC ranges from 37% (which appears to be an outlier) to 90% in 6 studies, with a median of 71%. In a study from Indiana University, there was no significant difference in OS (after salvage SBRT) between patients treated with prior conventionally fractionated radiation therapy and SBRT (median survival 25 vs. 13.4 months, P=0.28) (16).
Full table
Tumor control and survival outcome—SBRT after prior conventionally fractionated radiation
Several studies have analyzed outcomes in patients who had undergone SBRT after prior conventionally fractionated radiation for NSCLC. In a study from M. D. Anderson Cancer Center (MDACC) (17), 36 patients who had received conventionally fractionated radiation (median 61.5 Gy) for stage I–III NSCLC received salvage SBRT (mostly in 40–50 Gy in 4–5 fractions) 0–92 (median 22) months later for in-field (n=11) or out-of-field (n=25) recurrences. At a median follow-up of 15 months, the 2-year overall (OS) and progression-free survival (PFS) rates were 59% and 26% respectively, with a crude local control (LC) rate of 92%. Patients treated for isolated out-of-field recurrences without metastases had significantly (P=0.04) longer PFS than those treated for in-field recurrences or for out-of-field relapses with apparent distant or intra-thoracic metastases. In a subsequent MDACC study of 72 patients (57 with NSCLC), the 2-year OS and PFS were 74% and 42% respectively, with a crude LC rate of 98% (18).
In an Italian study of 17 patients with centrally recurrent or persistent Stage III NSCLC (13 within lung and 4 nodal) initially treated with 50–60 Gy in 20–30 fractions, SBRT (30 Gy in 5–6 fractions) was delivered 1–60 (median 18) months later (19). They reported a 2-year OS of 59%, 1-year LC of 86%, and 1-year distant control of 53%. Mayo Clinic analyzed 18 patients with 27 recurrent tumors (17 within the ipsilateral lung, 9 central in location) of whom 14 had stage II–III NSCLC; 2–113 months (median 18) after conventionally fractionated radiation, salvage SBRT was delivered, mostly 40–60 Gy in 3–5 fractions (20). The authors reported a 1-year OS of 88% and 2-year LC of 90%. In a study of SBRT for recurrent stage II–III NSCLC from University of Louisville, 27 patients (with 29 targets of which 17 were nodal) initially treated with conventionally fractioned radiation (median 64.8 Gy) received salvage SBRT of 30–54 Gy in 3–5 fractions after 3–113 (median 13) months (21). With a median follow-up of 12 months, the 2-year OS, PFS and local-PFS were 79%, 38% and 72% respectively. Adverse factors for LC included greater smoking pack-years and treatment for in-field recurrence. A higher biologically effective SBRT dose was associated with improved OS and PFS, and a longer interval between radiation courses was associated with improved OS. Georgetown recently published their outcomes on 5-fraction SBRT (25–45 Gy in 5 fractions) for ‘ultra-central’ recurrences (defined as parenchymal or nodal recurrences abutting the trachea, mainstem bronchus, or esophagus) after conventionally fractionated radiation (median 63 Gy) (22). With a median follow-up of 12 months, the 1-year OS and LC were 45% and 30%, respectively. An SBRT dose of ≥40 Gy was associated with a significantly higher 1-year LC (66.7% vs. 0% after a lower dose, P<0.01), as was longer duration between treatment courses. Higher dose was also associated with longer OS. A Turkish study of 28 patients with 34 targets from recurrent NSCLC showed that PFS and OS were not significantly impacted by the interval between prior radiation (conventionally fractionated in all but 1) and salvage SBRT (23).
Tumor control and survival outcome—SBRT after prior SBRT
Few studies have analyzed outcomes in patients who had undergone salvage SBRT after prior SBRT for NSCLC. A study from Karolinska University analyzed 29 patients with 32 tumors (11 of which were central) treated with SBRT (20–30 Gy in 1–2 fractions or 30–45 Gy in 3–5 fractions) for recurrence after prior SBRT (20–30 Gy in 2 fractions or 21–45 Gy in 3–5 fractions) for stage II–II NSCLC (n=10) or oligometastases (n=19) (24). At a median follow-up of 12 months, the 2-year OS was 43% with a crude LC rate of 52%. Two studies (with 9–10 patients each) also described outcomes of salvage SBRT after prior SBRT for any stage of NSCLC (25) or early stage NSCLC (26), with LC rates of 60–75%.
Prognostic factors in patients undergoing salvage SBRT
Two studies (26,27) have analyzed factors associated with outcomes in patients who had undergone SBRT after prior radiation, including those initially treated with SBRT and/or conventional radiation. A variety of dose-fractionation regimens were used for both courses of radiation in these studies. In a study from Wake Forest University of 33 patients with recurrent NSCLC (n=29) or SCLC (n=3), greater tumor size was significantly (P=0.03) associated with poorer LC (27); the interval between treatment courses was not a significant factor for LC or PFS, though OS was greater for patients with an interval >2 years (P=0.05). In a study from Drexel University, of 26 patients with 29 tumors (mostly from NSCLC), neither the biologically effective dose from the salvage SBRT course nor the target location (peripheral vs. central) significantly impacted LC (28). In a University of Pittsburgh Medical Center (UPMC) study of 72 patients with recurrent/residual (n=39) or new primary (n=33) NSCLC after prior radiotherapy, the 2-year OS and LC were 46% and 78% respectively. On multivariate analysis, a higher measured pre-treat positron emission tomography (PET) avidity significantly predicted for worse LC (P<0.001) and OS (P=0.016); greater target size (P=0.016) was also correlated with worse OS.
MDACC conducted a recently published phase II study of salvage SBRT (40–50 Gy in 4 fractions) after prior therapy for Stage I–III NSCLC (31); among the 59 enrolled patients, prior treatment included surgery (n=41) and/or radiotherapy (n=33). The authors did not analyze potential effects of prior treatment (i.e., resection vs. radiation therapy) on survival, tumor control or toxicity outcomes. In an exploratory analysis, tumor size, performance status, histologic subtype, pulmonary function, and peripheral blood neutrophil to lymphocyte ratio (NLR) were analyzed for OS and PFS. A greater tumor size (P=0.052) and higher post-SBRT NLR (P=0.065) were borderline significant for worse PFS, while a higher NLR (P=0.012) was associated with a significantly worse OS. The authors postulated that the NLR may be indicative of a poorer tumor immune response as a result of a relatively lower lymphocyte count.
Salvage SBRT treatment tolerance and toxicity
Tumor control and survival outcomes in the setting of re-irradiation need to be balanced against toxicity risks. A previous comprehensive review (4) summarizes the toxicity outcomes with SBRT for re-irradiation, and the factors associated with toxicity risks. Radiation-induced long toxicity (RILT) was reported as the most common adverse event, albeit with a wide range reported across studies (0–100% grade 2–3 RILT) due to the variability in patient and treatment factors across the different studies. There is a low, albeit not negligible, risk of fatal toxicity when SBRT is used in the re-irradiation setting (4).
One would assume dosimetric factors are significant for toxicity outcomes after salvage SBRT. Unexpectedly, based on extremely limited studies, dose-volume metrics of lung exposure, from either the SBRT or composite plans, correlated with lung toxicity risks in only one study (18) and not in 5 others (19-21,24,32) that studied dosimetric factors. These findings may reflect re-irradiated lung being less susceptible to radiation injury; dysfunctional lung may not lose function from additional damage. However, extreme caution is warranted on the specific types of organs (parallel versus series) being re-irradiated, and the location(s) of the treatments. Central (vs. peripheral) lung location was a significant factor in only 2 studies (24,29) and not others (17,20,21,30), perhaps resulting from more careful patient selection and/or conservative dose-fractionation with previously irradiated central locations. Composite normal tissue (e.g., lung, trachea-bronchial tree, large vessels, esophagus and chest wall) dose-volume measures could potentially be used to predict toxicity risks, but data on relatively safe organ at risk thresholds are lacking. In one study, overlapping retreatment fields correlated with greater risk of chest-wall toxicity (17), though composite chest wall exposure was not a significant factor in 2 other studies (20,33). It is important to note that the biologic effects on re-irradiation are largely unknown, and the data are extremely limited on the dosimetric effects on the outcome of SBRT for salvage.
SBRT for post-resection recurrences of NSCLC
For patients with parenchymal lung recurrences of NSCLC after resection, SBRT is a relatively minimally invasive definitive treatment approach that may be better suited to patients whose lung function and/or comorbidities preclude another thoracic surgery.
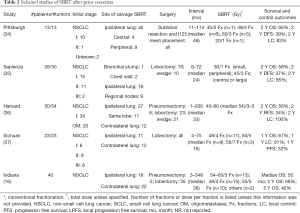
Table 2 summarizes selected studies of SBRT for salvage after prior thoracic resection for NSCLC. Fewer studies have specifically analyzed patients who underwent SBRT for new or recurrent NSCLC after prior resection. Following salvage SBRT, after prior resection, the 2-year OS ranged from 56% to 68% in 4 studies reviewed here, while the 2-year LC ranged from 83% to 100% in 3 of these studies (one of which included patients that received a brachytherapy mesh placement at time of initial treatment). SBRT as a salvage treatment is considered to be safe in this setting (16,34-37), although the toxicity data are limited. Grade 2 pulmonary symptoms ranged from 10% to 34% in 4 studies (34-37). In a study from Indiana University, there were no significant differences in OS (after salvage SBRT of 54–60 Gy in 3 fractions or 48 Gy in 4 fractions) between patients with prior pneumonectomy and lobectomy (median survival 56 vs. 50 months, P=0.58) (16).
Full table
Tumor control and survival outcome
UPMC analyzed outcomes in 13 early stage NSCLC patients who had undergone SBRT (mostly 45–60 Gy in 3–5 fractions) for recurrence after prior sublobar resection and I-125 mesh placement [a procedure that was not shown to improve LC in a recent American College of Surgeons Oncology Group study (38)]. The 2-year OS and disease-free survival were 66% and 39%, respectively, and the 2-year LC was 83% (34). No factors were found to be associated with LC, including the volume of planning target volume (PTV), biologically effective dose, time to recurrence after surgery or tumor location. An Italian study reviewed 28 patients with 30 tumors from recurrent NSCLC initially treated with lobectomy or wedge resection (35). Patients received SBRT in 1 fraction, 30 Gy for small peripheral lesions and 3 fractions to 45 Gy for large or central lesions. They reported similar outcomes to the UPMC group with 2-year OS, DFS and LC rates of 58%, 37% and 85%, respectively. The location of relapse and SBRT treatment (mediastinal nodes vs. ipsilateral lung/chest wall/bronchial stump) did not significantly impact OS, DFS, metastasis-free survival or LC.
In a study from Harvard University, 50 patients with 54 tumors from primary NSCLC or oligometastases underwent SBRT (40–60 Gy in 3–5 Fx) after prior pneumonectomy, lobectomy or wedge resection (36). Their survival outcomes were also similar to the 2 studies described above, with 2-year OS and recurrence-free survival rates of 56% and 34%, respectively, and LC of 100%. In a Chinese study, 23 patients underwent SBRT (48–56 Gy in 4–7 fractions) after recurrence of NSCLC post-lobectomy (37); the authors reported 1-year OS, PFS and LC rates of 67%, 32% and 91%, respectively.
Treatment tolerance and safety
Generally, SBRT in the salvage setting after prior resection appears to be well-tolerated. In the UPMC study of SBRT after prior sublobar resection and brachytherapy mesh (34), 2 patients (15%) developed grade 2 pulmonary symptoms and none developed grade ≥3 pulmonary toxicity. A patient with a central tumor that had also been treated with radiofrequency ablation had a grade 3 esophageal stricture. In the aforementioned study from Italy (35), grade 2–3 radiation pneumonitis occurred in 10% of patients, and late grade 2 fibrosis occurred in 7%. In the Chinese study discussed above (37), the rate of grade ≥2 radiation pneumonitis (occurring in 34%) was significantly associated with the volume of PTV (P=0.039) and ipsilateral lung volume receiving >5 Gy (P=0.034), though not other lung dosimetric parameters.
Comparative outcome between salvage SBRT after prior resection versus primary SBRT
For patients undergoing SBRT after prior resection, it is unclear if survival, tumor control outcomes or toxicity risks are different compared to those without prior resection. Conceptually, surgical disruption of tissue and tissue vasculature may affect tumor control or toxicity risks, though is difficult to study. In a Polish study of 61 patients treated with SBRT alone, SBRT for boost or SBRT for salvage after resection (39), those who underwent salvage therapy (n=20) experienced worse LC compared to those treated with SBRT alone or as a boost (27% vs. 63% and 54% respectively, P=0.02). The authors postulated that in the salvage setting, LC was compromised because more conservative dosing was used out of consideration of normal tissue tolerance. The study did not provide sub-grouped data on dose or dose fractionation and did not describe the interval from surgery in those 20 patients treated with salvage therapy.
In the aforementioned study from Harvard University (36), in addition to the 50 patients treated with SBRT after prior resection, 80 treated with SBRT without prior lung resection were also analyzed. On univariate analyses, prior lung resection for NSCLC recurrence was associated with significantly improved LC (100% vs. 85% at 2-year, P=0.03), but no impact was seen on survival, PFS or other cancer control outcomes. Comparing the toxicity risk between these groups, prior lung resection was associated with an appreciable and significant difference in grade ≥2 radiation pneumonitis (at 1-year, 12% vs. 1%, P<0.01) and any grade ≥2 toxicity (at 4-year, 16% vs. 1%, P=0.01). However, on multivariate analyses, prior lung resection was not significantly associated with grade ≥2 radiation pneumonitis. The study from Indiana University also showed no significant difference in survival after SBRT between patients after prior resection (n=40 patients) versus those without any prior treatment (n=243 patients) (16).
Systemic therapy in patients undergoing salvage SBRT for NSCLC recurrences
Data on the role of systemic therapy in the setting of localized, recurrent NSCLC treated with SBRT are lacking, as are evidence-based guidelines to inform decision-making. As such, a systematic review is presently not possible. The clinical decision on which (if any) systemic agents to consider should be individualized to the patient and impacted by prior use of systemic therapy (and timing of NSCLC recurrence relative to prior systemic therapy), extent and stage of disease at recurrence (i.e., American Joint Committee on Cancer: AJCC “r stage”), the NSCLC histology and molecular-pathologic characteristics, as well as the patient’s performance status and comorbidities.
Salvage thoracic radiation: radiation treatment planning considerations
When planning for SBRT salvage therapy, the most conservative approach would be to generate composite doses from both treatment courses and meet the dose limits of OARs as if the treatments were delivered at the same time. For patients with prior radiotherapy, published thoracic OAR dose-constraints for SBRT are lacking (4) with the possible exception of re-irradiation spinal cord/spinal canal tolerance (40,41). Thus, every effort should be made to minimize normal tissue exposure in the re-irradiation setting, utilizing technologies such as more rigorous motion management, intensity-modulated radiation therapy/volume-modulated arc therapy (IMRT/VMAT) and functional avoidance treatment planning. Notably, IMRT/VMAT increases the monitor unit delivery, which increases the integral dose exposure (i.e., increases the low to moderate dose exposure), and generally delivers a more homogeneous (relative to static beams or arcs) target dose. When using IMRT/VMAT planning for SBRT, we recommend efforts to create dose gradients (i.e., sharper dose fall-offs) into the treatment plan, which could be accomplished by prioritizing adjacent normal tissue sparing and/or creating a simultaneous boost PTV with negative margins (i.e., small internal volume that receives a higher dose than the target periphery). Lung functional imaging, like ventilation/perfusion single photon emission tomography (V/Q SPECT), may be used to map the lung function and guide functional lung-avoiding SBRT planning. The target dose, dose fractionation and dosimetric limits of OARs should be individualized based upon the prior dose exposure to the target and OARs, with the goal of optimizing the ratio of tumor control and normal tissue toxicity. For bulkier recurrences, or when the OAR relative dose exposure is high, conventional fractionation, or hypofractionation (at lower fractional doses than SBRT) might be preferred over SBRT. Conventionally fractionated radiotherapy, with or without concurrent chemotherapy, can also be considered for salvage. During the pre-SBRT era, a study from University of Michigan reported results of concurrent chemoradiation for recurrent NSCLC after resection and generated comparable results to that of newly diagnosed stage III NSCLC (42). In general, patients should be informed of the uncertain nature and limited knowledge of long-term treatment outcomes.
Conclusions
Based upon the studies reviewed, SBRT has generated a reasonable rate of 2-year survival and tumor control in the setting of salvage therapy after prior radiotherapy and/or prior resection. The compilation of data is somewhat limited by the wide variety in patient clinico-pathological and treatment characteristics. Toxicity risks in the SBRT re-irradiation setting are acceptable albeit with appreciable risks of severe to potentially fatal toxicity (4), necessitating a careful estimation of risks vs. benefits of SBRT. There are fewer studies published on the use of SBRT after prior resection. SBRT in the salvage setting after prior resection appears to be well-tolerated with excellent survival outcomes. RILT and other toxicity risks are comparable to those reported in historical patients treated with SBRT alone (i.e., SBRT without prior resection, which is not reviewed here), albeit with only 1 study in this review that made such a comparison.
Notably, the data on salvage SBRT are limited, due to the retrospective nature of published studies (and all but 4 with <40 patients), with various clinical scenarios (i.e., original NSCLC stage, prior treatment, size/volume and location of target amenable to salvage SBRT) and a range of SBRT dosing and techniques. From the published reports to date, most patients have received ~40–60 Gy in 3–5 fractions. In those patients with peripheral lung tumors and/or without prior radiotherapy, 50–60 Gy in 3–5 fractions is a reasonable approach based upon published data. Central lesions, particularly in the re-irradiation setting, may be better suited for less aggressive SBRT dose-fractionation schedules such as 40–50 Gy in 5 fractions, or (particularly for bulkier lesions) more protracted courses such as 10+ fraction hypofractionated image-guided radiation therapy or conventionally fractionated radiation. More studies are needed to better understand the tumor control, survival and toxicity of SBRT for salvage therapy of NSCLC patients, as well as the potential prognostic factors that could affect these outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lo SS, Loblaw A, Chang EL, et al. Emerging applications of stereotactic body radiotherapy. Future Oncol 2014;10:1299-310. [Crossref] [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Milano MT, Constine LS, Okunieff P. Normal tissue toxicity after small field hypofractionated stereotactic body radiation. Radiat Oncol 2008;3:36. [Crossref] [PubMed]
- Milano MT, Mihai A, Kong FM. Review of thoracic reirradiation with stereotactic body radiation therapy: A focus on toxicity risks. Pract Radiat Oncol 2018;8:251-65. [Crossref] [PubMed]
- Zhao J, Yorke ED, Li L, et al. Simple Factors Associated With Radiation-Induced Lung Toxicity After Stereotactic Body Radiation Therapy of the Thorax: A Pooled Analysis of 88 Studies. Int J Radiat Oncol Biol Phys 2016;95:1357-66. [Crossref] [PubMed]
- Kimsey F, McKay J, Gefter J, et al. Dose-Response Model for Chest Wall Tolerance of Stereotactic Body Radiation Therapy. Semin Radiat Oncol 2016;26:129-34. [Crossref] [PubMed]
- Kang KH, Okoye CC, Patel RB, et al. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers (Basel) 2015;7:981-1004. [Crossref] [PubMed]
- Duijm M, Tekatli H, Oomen-de Hoop E, et al. Esophagus toxicity after stereotactic and hypofractionated radiotherapy for central lung tumors: Normal tissue complication probability modeling. Radiother Oncol 2018;127:233-8. [Crossref] [PubMed]
- Verstegen NE, Maat AP, Lagerwaard FJ, et al. Salvage surgery for local failures after stereotactic ablative radiotherapy for early stage non-small cell lung cancer. Radiat Oncol 2016;11:131. [Crossref] [PubMed]
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010;5:2003-7. [Crossref] [PubMed]
- Hamaji M, Chen-Yoshikawa TF, Matsuo Y, et al. Salvage video-assisted thoracoscopic lobectomy for isolated local relapse after stereotactic body radiotherapy for early stage non-small cell lung cancer: technical aspects and perioperative management. J Vis Surg 2017;3:86. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Treatment and Prognosis of Isolated Local Relapse after Stereotactic Body Radiotherapy for Clinical Stage I Non-Small-Cell Lung Cancer: Importance of Salvage Surgery. J Thorac Oncol 2015;10:1616-24. [Crossref] [PubMed]
- Chen F, Matsuo Y, Yoshizawa A, et al. Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients. J Thorac Oncol 2010;5:1999-2002. [Crossref] [PubMed]
- Antonoff MB, Correa AM, Sepesi B, et al. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J Thorac Cardiovasc Surg 2017;154:689-99. [Crossref] [PubMed]
- Allibhai Z, Cho BC, Taremi M, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Respir J 2012;39:1039-42. [Crossref] [PubMed]
- He C, Liu YM, Cerra-Franco A, et al. Long-term survival after salvage SBRT for recurrent or secondary non-small cell lung cancer after prior surgery or radiation therapy. J Clin Oncol 2018;36:abstr 8558.
- Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys 2010;78:1387-93. [Crossref] [PubMed]
- Liu H, Zhang X, Vinogradskiy YY, et al. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:1017-23. [Crossref] [PubMed]
- Trovo M, Minatel E, Durofil E, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;88:1114-9. [Crossref] [PubMed]
- Owen D, Olivier KR, Song L, et al. Safety and Tolerability of SBRT after High-Dose External Beam Radiation to the Lung. Front Oncol 2015;4:376. [Crossref] [PubMed]
- Parks J, Kloecker G, Woo S, et al. Stereotactic Body Radiation Therapy as Salvage for Intrathoracic Recurrence in Patients With Previously Irradiated Locally Advanced Non-Small Cell Lung Cancer. Am J Clin Oncol 2016;39:147-53. [Crossref] [PubMed]
- Repka MC, Aghdam N, Kataria SK, et al. Five-fraction SBRT for ultra-central NSCLC in-field recurrences following high-dose conventional radiation. Radiat Oncol 2017;12:162. [Crossref] [PubMed]
- Ceylan C, Hamaci A, Ayata H, et al. Re-Irradiation of Locoregional NSCLC Recurrence Using Robotic Stereotactic Body Radiotherapy. Oncol Res Treat 2017;40:207-14. [Crossref] [PubMed]
- Peulen H, Karlsson K, Lindberg K, et al. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol 2011;101:260-6. [Crossref] [PubMed]
- Valakh V, Miyamoto C, Micaily B, et al. Repeat stereotactic body radiation therapy for patients with pulmonary malignancies who had previously received SBRT to the same or an adjacent tumor site. J Cancer Res Ther 2013;9:680-5. [Crossref] [PubMed]
- Hearn JW, Videtic GM, Djemil T, et al. Salvage stereotactic body radiation therapy (SBRT) for local failure after primary lung SBRT. Int J Radiat Oncol Biol Phys 2014;90:402-6. [Crossref] [PubMed]
- Kilburn JM, Kuremsky JG, Blackstock AW, et al. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol 2014;110:505-10. [Crossref] [PubMed]
- Patel NR, Lanciano R, Sura K, et al. Stereotactic body radiotherapy for re-irradiation of lung cancer recurrence with lower biological effective doses. J Radiat Oncol 2015;4:65-70. [Crossref] [PubMed]
- Binkley MS, Hiniker SM, Chaudhuri A, et al. Dosimetric Factors and Toxicity in Highly Conformal Thoracic Reirradiation. Int J Radiat Oncol Biol Phys 2016;94:808-15. [Crossref] [PubMed]
- Horne ZD, Dohopolski MJ, Clump DA, et al. Thoracic re-irradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation field. Pract Radiat Oncol 2018;8:e117-23. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki R, et al. Long-Term Outcomes of Salvage Stereotactic Ablative Radiotherapy for Isolated Lung Recurrence of Non-Small Cell Lung Cancer: A Phase II Clinical Trial. J Thorac Oncol 2017;12:983-92. [Crossref] [PubMed]
- Reyngold M, Wu AJ, McLane A, et al. Toxicity and outcomes of thoracic re-irradiation using stereotactic body radiation therapy (SBRT). Radiat Oncol 2013;8:99. [Crossref] [PubMed]
- Lester SC, Kilburn JM, Lucas JT, et al. An Evaluation of Toxicity Using Accumulated Total Dose Based on EQD2 for Thoracic Reirradiation Incorporating at Least 1 Course of SBRT. Int J Radiat Oncol Biol Phys 2014;90:S640-1. [Crossref]
- Gill BS, Clump DA, Burton SA, et al. Salvage stereotactic body radiotherapy for locally recurrent non-small cell lung cancer after sublobar resection and i(125) vicryl mesh brachytherapy. Front Oncol 2015;5:109. [Crossref] [PubMed]
- Agolli L, Valeriani M, Carnevale A, et al. Role of salvage stereotactic body radiation therapy in post-surgical loco-regional recurrence in a selected population of non-small cell lung cancer patients. Anticancer Res 2015;35:1783-9. [PubMed]
- Hou Y, Hermann G, Lewis JH, et al. Clinical Outcomes After Lung Stereotactic Body Radiation Therapy in Patients With or Without a Prior Lung Resection. Am J Clin Oncol 2018;41:695-701. [Crossref] [PubMed]
- Xiong W, Xu Q, Xu Y, et al. Stereotactic body radiation therapy for post-pulmonary lobectomy isolated lung metastasis of thoracic tumor: survival and side effects. BMC Cancer 2014;14:719. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol 2014;32:2456-62. [Crossref] [PubMed]
- Dworzecki T, Idasiak A, Sygula D, et al. Stereotactic radiotherapy (SBRT) as a sole or salvage therapy in non-small cell lung cancer patients. Neoplasma 2012;59:114-20. [Crossref] [PubMed]
- Myrehaug S, Sahgal A, Hayashi M, et al. Reirradiation spine stereotactic body radiation therapy for spinal metastases: systematic review. J Neurosurg Spine 2017.428-35. [PubMed]
- Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:107-16. [Crossref] [PubMed]
- Cai XW, Xu LY, Wang L, et al. Comparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1100-5. [Crossref] [PubMed]


