Multidisciplinary quality improvement initiative to standardize reporting of lung cancer screening
Introduction
In 2014, the US Preventive Services Task Force issued a Grade B recommendation for annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) for patients with at least a 35 pack-year smoking history (1). As part of a high-quality LCS program, the lung nodules that are found during screening should be systematically categorized, documented, and managed (2). Without institutional adoption of a particular nodule classification system, reporting can differ across radiologists and result in variation in follow-up care pathways (3).
One such classification system is the Lung Imaging Reporting and Data System (Lung-RADSTM), which categorizes findings by likelihood of cancer (4). The Lung-RADS reporting system includes clear follow-up screening schedules for benign or negative results, provides surveillance plans and nodule management strategies for low-risk nodules, and imaging and biopsy for highly suspicious nodules (4,5). Successful implementation of a standardized process for reporting and tracking lung nodules can be challenging because it requires multidisciplinary buy-in, electronic health record (EHR) system changes, and workflow modifications.
As part of a larger 1-year, institutionally-funded quality improvement (QI) project (6), we aimed to address the lack of standardized processes for reporting and tracking screen-detected nodules within one academic medical center.
Methods
Setting and existing processes
This QI project involved workflow interventions in the Division of Cardiothoracic Imaging and the Multidisciplinary Thoracic Oncology Program (MTOP). The Division of Cardiothoracic Imaging is comprised of 5 attending radiologists and 1 fellow. After determining the primary indication for the CT scan, chest radiologists dictate findings into a text-based, structured report with pre-populated section headings. Attending radiologists sign off on all final reports. MTOP includes specialists in pulmonary medicine, thoracic surgery, medical and radiation oncology, thoracic radiology, pathology, and oncology nursing. MTOP specialists meet weekly to discuss complex findings and develop a care plan for each referral. Systems changes for this QI project were implemented in Agfa Talk v4.3 voice recognition software and our EHR system, Epic®.
QI project description
Processes and results to address key quality gaps in the primary care setting are reported elsewhere (6). In spring of 2015, we convened a multidisciplinary team (consisting of 2 primary care liaisons, 1 pulmonologist, 2 thoracic radiologists, 1 epidemiologist, and several health services researchers and coordinators) to focus on lung nodule categorization and follow-up on a larger health system level. The project ran from July 2015 to June 2016.
The team developed a project charter to guide its work, describe activities within the scope of the project, and define outcomes. At the project kickoff, all team members were oriented to the LCS process and a key driver diagram was developed (Figure 1 depicts the quality gap described in this report). Our main aim was to improve the delivery of appropriate LCS by obtaining multidisciplinary commitment to systematically use Lung-RADS. Our goal was to achieve a Lung-RADS documentation rate of at least 50% of all LCS LDCTs read by radiologists. The Institutional Review Board of the University of North Carolina determined that this project did not require its approval.
Categorization and documentation of LCS findings
Prior to project initiation, discussions regarding the need to implement Lung-RADS categorization for screening LDCTs were underway as part of an existing LCS registry initiative (7). The team agreed that Lung-RADS would facilitate reporting and communication regarding follow-up recommendations for screen-detected nodules. Three subspecialty team members obtained institutional buy-in for implementing Lung-RADS through Grand Rounds presentations. With this buy-in, and leadership support from the Executive Vice Chairman of Radiology, the group elected to implement Lung-RADS.
Next, the team worked on improving the radiologic dictation and reporting template used to record LDCT findings. The original dictation template provided a text-based structure for the report, but did not contain a section header for Lung-RADS documentation and recommended follow-up. We tested the use of a Lung-RADS section header to improve fidelity of documentation and promote standardization, similar to an existing process for documenting mammograms at this institution (8). The dictation templates were modified by adding a “Lung-RADS Category” section header to prompt interpreting radiologists to include a Lung-RADS category, and “Follow-Up Recommendation” as a second header to include the recommended action (e.g., repeat scan in 12 months). Subsequently, the Division Chief sent a memo to attending radiologists and fellows, and asked that each LCS LDCT receive a Lung-RADS assessment.
Clarification of follow-up care pathway
During team meetings, primary care members planned for appropriate follow-up care pathways for screening LDCTs. Once Lung-RADS was selected as the standard categorization and reporting system, the working group began to develop and test a category-specific follow-up care algorithm for screen-detected nodules and/or repeat annual screenings.
As part of the audit and feedback process, we discussed specific cases in which Lung-RADS assessments were missing or follow-up care recommendations were unclear.
Evaluation
We evaluated the categorization, documentation, and follow-up of LCS LDCTs through quarterly measurement of the proportion of LCS LDCTs that included a Lung-RADS assessment. To assess fidelity of documentation of Lung-RADS categorization, we developed a monthly report that identified screening LDCTs and extracted accompanying free-text impressions from radiologists’ dictated reports. The team developed an algorithm to analyze extracted impressions and provide frequency of Lung-RADS categorization and follow-up care recommendations. Team members who were representatives of the cardiothoracic imaging group received monthly feedback on the rate of Lung-RADS use with data at the group and individual radiologist level.
Results
Categorization and documentation of LCS findings
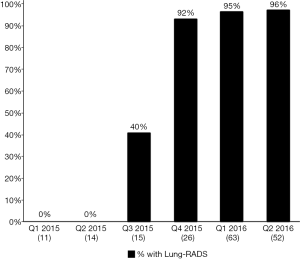
Figure 2 depicts the proportion, by quarter, of LCS LDCT reports that included a Lung-RADS assessment. In Q1- and Q2-2015, prior to the project onset, 0% of LCS LDCTs contained a Lung-RADS assessment. Lung-RADS categorization for screening LDCT examinations was implemented in July 2015. In January 2016, the Lung-RADS classification field was added to the radiology report template. By the end of Q1-2016, 94% of LDCTs performed for LCS contained a Lung-RADS assessment and a recommended follow-up action as outlined by the Lung-RADS care pathway (Figure 3). Radiologists also began including standardized clinical follow-up recommendations in addition to their image interpretations (e.g., “Lung-RADS 2, screen again in 12 months” vs. “Patient has 2 mm ground glass opacity”).
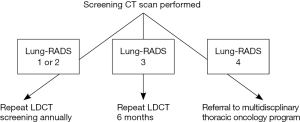
Follow-up care pathway
For the 3 quarters of 2015 and 2016 in which Lung-RADS was documented with >90% fidelity, the majority of those screened (n=141) had a Lung-RADS 1 (44%) or 2 (38%), which are negative or benign results (Table 1). All 12 LDCT screening examinations with a Lung-RADS 4 received multidisciplinary follow-up. Of those 12 examinations, 9 were followed with serial imaging only, 2 underwent CT-guided biopsy (1 finding carcinoma-in-situ and the other metastatic prostate cancer), and 1 underwent right upper lobe lobectomy with pathology showing stage 1a adenocarcinoma (negative nodes, and clear margins).
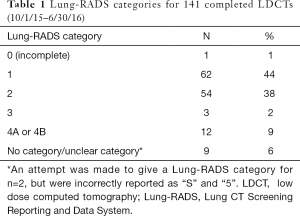
Full table
Discussion
We developed systematic processes for lung nodule categorization and documentation using Lung-RADS, reached consensus among subspecialists and primary care providers regarding appropriate follow-up care pathways, and achieved successful implementation and Lung-RADS-based management of lung nodules identified through screening LDCTs. We operationalized a system of standardized radiologic reporting of screen-detected nodules with greater than 90% fidelity (5). All “suspicious” nodules (Lung-RADS 4) received appropriate follow-up.
Our implementation of the Lung-RADS nodule classification system was successful. Through institutional endorsement and simple, system-level changes in the dictation template for thoracic radiology, we were able to achieve high fidelity in documentation of Lung-RADS. Lung-RADS can facilitate a clinical action plan salient for both primary care physicians and subspecialists, and clarify responsibility for patient follow-up based on nodule classification.
Our project has limitations. First, our program was conducted at a single academic medical center and may not be generalizable to other institutions or implementation contexts. Second, the absence of a discrete Lung-RADS category field in our EHR precluded our ability to develop a more fully-automated system to generate reminders. However, recent developments in EHR software are increasingly making such automation feasible. Third, this project focused on structured reporting, a single component of a much larger and more complex LCS process (2). Finally, we did not collect detailed demographic or risk factor data on patients undergoing screening, limiting our ability to draw comparisons between our population and those in the National Lung Screening Trial.
Our project demonstrates how a multidisciplinary QI effort that crosses primary care, pulmonary, and thoracic radiology can improve structured reporting within the LCS cascade.
Acknowledgements
The authors acknowledge Laura Brown, MPH, John Eick, MD, Mikael Anne Greenwood-Hickman, MPH, Samuel S. Weir, MD, Teri Malo, PhD, Annie Whitney, MS, PMP and her team at UNC Practice Quality and Innovation Population Health group, the UNC Primary Care Improvement Collaborative, and the UNC Institute for Healthcare Quality Improvement.
Funding: This work was supported by grants from the UNC Institute for Healthcare Quality Improvement; Department of Medicine at the University of North Carolina; and National Institutes of Health/National Cancer Institute (R21CA175983 to LM Henderson).
Footnote
Conflicts of Interest: M Pignone was a member of the US Preventive Services Task Force; the other authors have no conflicts of interest to declare.
Disclaimer: The views presented here are not necessarily those of the USPSTF, nor do they reflect the official policy of the National Institutes of Health.
References
- Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Wiener RS, Gould MK, Arenberg DA, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med 2015;192:881-91. [Crossref] [PubMed]
- Pinsky PF, Gierada DS, Nath PH, et al. National lung screening trial: variability in nodule detection rates in chest CT studies. Radiology 2013;268:865-73. [Crossref] [PubMed]
- American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS) [Internet]. Silver Spring, MD; 2014. Available online: http://www.acr.org/Quality-Safety/Resources/LungRADS
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- Brenner AT, Cubillos L, Birchard K, et al. Improving the Implementation of Lung Cancer Screening Guidelines at an Academic Primary Care Practice. J Healthc Qual 2018;40:27-35. [PubMed]
- Henderson L, Molina P, Birchard K, et al. Development Of A Lung Cancer Screening Registry. Am J Respir Crit Care Med 2016;193:A1283.
- Sickles EA, D'Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography, 5th ed. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology, 2013.



