
The evolving toxicity profile of SBRT for lung cancer
Introduction
Stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), is a commonly used technique for early stage non-small cell lung cancer (NSCLC). Reported toxicities of SBRT including pneumonitis, chest wall pain, rib fracture, esophagitis and brachial plexopathy have previously been described (1,2). We aim to review the current literature to identify risk factors for development of chest wall toxicity and to characterize toxicity associated with SBRT to central and ultra-central tumors. In addition, we examine the literature regarding the potential adverse effects associated with SBRT in conjunction with immunotherapy or as an additional boost following definitive chemoradiotherapy.
Chest wall/rib fracture
Toxicity involving the chest wall, including chest wall pain and/or rib fracture, is a known potential adverse effect of SBRT to peripheral lung lesions. This is typically a late adverse treatment effect; median onset of chest wall pain ranges from 6–9 months following treatment (3-7) and median time to rib fracture ranges from 13–22 months post treatment (8-11). However, the reported incidence of chest wall toxicity following SBRT varies widely from 8–46% (9). An example of a rib fracture after SBRT is presented in Figure 1.
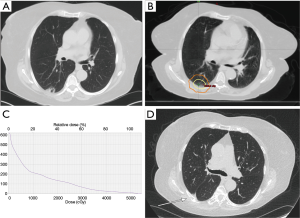
Attempts to predict the incidence of chest wall toxicity have been well documented, with varied results. Prior reports have estimated a threshold of V30 Gy <30 cc to the chest wall to reduce the risk of toxicity (7), while another study found V30 Gy <70 cc to be predictive when the contoured volume width was 2 cm from the lung (12). A more recent study found that although V30 Gy was initially predictive of grade ≥2 chest wall pain on univariate analysis, only tumor size and maximum dose received by 1 cc (Dmax 1 cc) of the chest wall was significant on multivariate analysis (3) and another, utilizing machine learning to identify prognostic factors, identified rib dose to 1 cc <40 Gy and a more conservative chest wall dose of 30 cc <19 Gy to prevent this adverse effect. The authors also note that their cut offs are most applicable to treatment with 10 Gy ×5 or 12.5 Gy ×4 which occurred most frequently in their dataset (13). Variability in reports may be due in part to multiple definitions of chest wall toxicity reported, including pain and/or rib fracture. A study from the University of Toronto suggests that rib fractures and chest wall pain are two separate entities with likely separate etiologies and should therefore be considered as such. They reported on 289 tumors treated to a dose of 48–60 Gy in 4 fractions with median follow-up of 21 months. Symptomatic chest wall pain was seen in 16% of tumors treated; 48% with symptomatic rib fracture and 52% without fracture. Rib fractures were noted in 17% of tumors treated; 56% asymptomatic and 44% symptomatic. Tumor location adjacent to the chest wall was a significant predictor of rib fracture on multivariate analysis but no predictors for chest wall pain without fracture were identified (9).
In contrast, a Korean group recently described their experience using CyberKnife® for thoracic SBRT, with median follow up of 26.7 months. In this series, 16.6% of patients experienced rib fracture in 3 years; 63% of which were asymptomatic. Three patients initially reported grade 1 chest wall pain at 2–3 months post-treatment without any evidence of rib fracture. After longer follow-up, however, all 3 patients experienced symptomatic rib fractures. The authors suggest that tumor distance of ≤0.4 cm and 2 Gy equivalent dose (EQD2) >140 Gy received by 4.6 cc of the rib were significant risk factors for radiation induced rib fracture (8). While a high dose delivered to a small volume of the rib is most predictive of an increased risk of rib fracture, there is variability in the dose-volume parameters that have been described in the literature. The dose received to a volume of 4.6 cc (D 4.6 cc), 2 cc (D 2 cc), 0.5 cc (D 0.5 cc) of the rib and maximum dose (Dmax) to the rib have all been associated with increased risk of rib fracture (8,10,14,15).
Advancements in automated contouring have allowed for the analysis of a greater number of ribs in a more expeditious manner. A recent analysis by Stam et al. utilized atlas-based automatic segmentation for 466 patients treated with SBRT. Their analysis revealed 9% of patients with grade ≥2 rib fractures. Dmax, age and BMI were significantly associated with incidence of rib fracture on multivariate analysis. Dmax was chosen to create a Normal Tissue Complication Probability (NTCP) model, which included time to toxicity, to predict the incidence of rib fracture in their cohort. They found that the risk of grade ≥2 rib fracture was <5% when the EQD2 Dmax was <225 Gy (11).
A study from Wake Forest University also utilized automated segmentation and reported a dose-dependent incidence of early (3 months) cortical bone thinning in regions of ribs receiving ≥10 Gy after SBRT. Their study suggests that cortical bone thinning due to high doses may be a mechanism by which these rib fractures occur (16). The mechanism for chest wall pain in the absence of rib fracture, however, remains unclear. Some reports have suggested peripheral nerve damage as a primary mechanism (4,12). Welsh et al. found that an elevated BMI (≥29 kg/m2) was a strong predictor for chest wall pain after SBRT on univariate analysis, with a trend to increased incidence in diabetic patients in this population. As peripheral neuropathy is a known complication of uncontrolled diabetes, it is speculated that mechanisms of nerve toxicity in the setting of high glucose levels may play a role in this observed association (5,11). Additional prospective analysis would be required to further explore this possibility.
Central/ultra-central tumors
An early prospective trial reported an increased risk of treatment-related toxicities and mortalities for central tumors treated with SBRT, which led to a recommendation of a 2 cm “no-fly” zone surrounding the proximal bronchial tree (PBT) (17). More recently, efforts have been made to further sub-classify this region, such as the definition of “ultra-central” tumors as GTV or PTV that directly abuts or overlaps the trachea or PBT (18,19).
A Phase II clinical trial from Washington University reported that 11 Gy ×5 was a tolerable dose for central lung tumors (20). Forty-one patients were eligible for toxicity evaluation, and 14.6% had grade 3 or worse late toxicity including one case of fatal hemoptysis in a tumor involving the pulmonary artery.
RTOG 0813 was a phase I/II study designed to investigate the maximally tolerated dose (MTD) and efficacy of SBRT to centrally located lesions, defined as “within or touching the zone of the proximal bronchial tree or adjacent to mediastinal or pericardial pleura” (21). Patients were treated in 5 fractions to doses escalating from 50–60 Gy. There was also a contingency for dose de-escalation in case of significant toxicity. There were 5 dose-limiting toxicities (DLTs); grade ≥3 hypoxia, pneumonitis, bradycardia and death. Fatal adverse events were seen in one patient treated to 52.5 Gy, two patients treated to 57.5 Gy and one patient treated to 60 Gy. There were no fatal events in the group treated to 50 Gy (22). Preliminary analysis reported MTD of 60 Gy in 5 fractions, associated with a 7.2% rate of DLT (21).
Stam et al. stratified patients by shortest distance from the edge of the GTV to the PBT. Cohorts were stratified by distances of >2 cm (peripheral), 1–2 cm and <1 cm from the PBT. The study evaluated the incidence of non-cancer related deaths of 769 patients from 5 institutions treated with SBRT to central lesions to a median dose of 3×18 Gy (range, 18–64 Gy in 1–10 fractions). A statistically significant difference in non-cancer related death between tumors <1 cm and >2 cm away from the PBT was found, with no difference between tumors 1–2 cm and >2 cm from the PBT (23). Although specific toxicity was not clearly identified in their report, it is possible that treatment related toxicity plays a significant role in the rate of non-cancer related deaths observed.
Ultra-central tumors which abut or invade the trachea/PBT, or are immediately adjacent to the esophagus, present a challenge for treatment. Retrospective analyses of toxicity following SBRT to tumors in this location have reported mixed results. A study from Stanford University described their experience treating peripheral, central and ultra-central tumors with SBRT. Sixty-eight patients, 34 with peripheral tumors and 34 with central tumors, including 7 which were ultra-central, were treated to a dose of 50 Gy in 4–5 fractions, with a median follow-up of 24.1 months. There was one grade 4 event (pneumonitis) in the central group and one grade 3 event (chest wall pain) in the peripheral group. No instances of brachial plexopathy, hemoptysis, hemorrhage, spinal cord injury or death were reported. Interestingly, there were no ≥ grade 2 toxicities seen in the 7 patients in the ultra-central group (14). In contrast, a group at Memorial Sloan-Kettering Cancer Center reported an 8% incidence of ≥ grade 3 adverse events, including two deaths, in their cohort of 125 patients. Adverse events included respiratory, gastrointestinal and cardiac events. One patient with a tumor abutting the esophagus developed esophagitis which progressed further to a fistula. Another experienced upper GI bleeding requiring endoscopic intervention. The two fatalities reported involved a patient with tumor encasing the left superior bronchus who experienced fatal hypoxemia 2 weeks following the completion of treatment and a second patient with pneumonia requiring intubation and who developed subsequent fatal hemoptysis 7 months after treatment (24).
Haseltine et al. described their experience with fatal respiratory complications following SBRT to central (≤1 and >1 cm from PBT) and ultra-central tumors. With a median follow-up of 22.7 months, the overall rate of grade ≥3 toxicity was 12%, including 4 SBRT related deaths. All four deaths occurred in the ultra-central group, within one year of treatment. Treatment related fatalities included pneumonia leading to sepsis, pneumonia leading to acute respiratory failure and two patients with acute pulmonary hemorrhage. The observed toxicity might have been related to the use of VEGF inhibitors for some patients. Tumors located ≤1 cm from the PBT had significantly more ≥ grade 3 toxicity (30.7%) compared to the rest of the cohorts. The authors suggest that proximity to the PBT may better predict the risk of toxicity for this population and that anti-VEGF treatment in close temporal proximity before or after SBRT may be an additional risk factor (25).
The use of single fraction SBRT to centrally located tumors has not been widely reported. Ma et al. described their experience with single fraction SBRT to central lung tumors. Eleven patients were treated with a single fraction to a dose of 26–30 Gy, with a median follow up of 12 months. Two patients experienced grade 3–4 toxicity: one patient experienced grade 3 vocal cord palsy and another experienced grade 4 bronchopulmonary hemorrhage and eventually died after pneumonectomy.
The use of SBRT for mediastinal nodal metastases has also not been described well in the literature. In a retrospective study, investigators from the University of Pittsburgh Medical Center described their experience with SBRT to isolated hilar and mediastinal nodes or isolated oligo-recurrence. With a median follow up of 16.4 months in a mixed population of patients with prior surgery, radiation or chemotherapy, 45% of patients experienced no acute toxicity, 45% experienced grade 1 toxicity and one patient transiently experienced grade 2 esophagitis. There were 3 cases (7.5%) of grade 3–5 toxicity. Late grade 1 toxicity (asymptomatic radiation pneumonitis, scant hemoptysis, and dyspnea) was seen in 33% of patients. One patient died 7.5 months after SBRT following recurrent tumor eroding into the airway causing massive hemoptysis. No other late grade ≥3 toxicities were reported (26). The authors conclude that their data support safety of the use of SBRT for hilar and mediastinal lymphadenopathy, although they acknowledge that toxicity could be underreported due to short follow up interval and the overall poor prognosis of the study population.
The use of SBRT in the central portion of the thorax continues to be investigated and may eventually be further classified to include central versus ultra-central location and primary versus nodal disease, as well as which critical structure the tumor abuts (esophagus, PBT, great vessel, etc.). Reports of toxicity vary and, therefore, continued caution must be used when treating these centrally located lesions. The American Society of Clinical Oncology (ASCO) endorsement of the American Society of Radiation Oncology (ASTRO) guidelines for SBRT in early stage NSCLC recommends that the use of 4–5 fractions for centrally located tumors may reduce the risk of severe toxicity. Additionally, they discourage SBRT to ultra-central tumors and instead recommend either hypofractionated radiation in 6–15 fractions or conventionally fractionated radiation in 2 Gy fractions (22,27). Results from prospective trials such as RTOG 0813 and the SUNSET trial evaluating the MTD of SBRT in ultra-central tumors (NCT03306680) may provide guidance regarding the safe utilization of SBRT in this context (28).
Immune checkpoint inhibitors
The use of immune checkpoint inhibitors (ICPI) such as PD-1 and PD-L1 inhibitors continues to grow in the treatment of NSCLC. FDA approved ICPIs include PD-1 inhibitors nivolumab and pembrolizumab and PD-L1 inhibitor atezolizumab for metastatic NSCLC. Pembrolizumab has also been approved in conjunction with chemotherapy for patients with untreated metastatic NSCLC and ≥50% PD-L1 expression. More recently, durvalumab, a PD-L1 inhibitor, has been approved for unresectable stage III NSCLC with disease that has not progressed following definitive concurrent chemoradiation therapy (29,30).
Documented adverse effects of ICPIs include fatigue and GI-related and respiratory-related events. Reported rates of any grade ≥3 ICPI-related toxicity range from 7–17%. The incidence of pulmonary-related events of any grade range from 7–10%. Grade ≥ 3 pneumonitis has been reported in 0–3% of cases, although this includes isolated incidences of grade 5 pneumonitis and hypoxic pneumonia (31-36). Naidoo et al. reported results of a retrospective review of pneumonitis following treatment with PD-1/PD-L1 inhibitors either as monotherapy or in conjunction with CTLA-4 inhibitors in 915 patients at two institutions. Grade ≥3 pneumonitis was identified in 12 patients (1.3%), all of whom required hospitalization. There were no grade 5 events. The authors also noted that the onset of pneumonitis was earlier in patients treated with combination therapy compared to those on ICPI alone (37).
There has been interest in combining ICPIs with SBRT in order to produce an abscopal response, or a treatment effect outside of the region receiving radiation. Pre-clinical studies have revealed relationships between tumors and the immune system which may be enhanced by radiation (38). One case report documented a dramatic tumor regression of additional lesions not treated with radiation in a patient with metastatic melanoma receiving SBRT and ipilimumab (39). Given the independent adverse effects associated with ICPIs and SBRT, there may be concern that the combination of these treatment modalities may have an additive effect on potential toxicities. There are few reports to date describing the effects of the combination of thoracic SBRT and ICPIs. One retrospective study from the Moffitt Cancer Center, presented in abstract form, reported their experience with 29 patients receiving thoracic radiation therapy within 6 months before or after receiving ICPI therapy. Treatment regimens included both SBRT and conventional fractionation treatments ranging from 10–70 Gy in 1–35 fractions. Two patients developed immunotherapy-related pneumonitis prior to the initiation of radiation, and there was one possible grade 5 toxicity in a patient who received 20 Gy in 5 fractions to the right hilum/lung, initiated one month following the last dose of anti PD-1 therapy (40). As prospective data are not yet available, caution must be taken when treating with a combination of thoracic SBRT and ICPIs. There are a number of ongoing and upcoming clinical trials designed to further evaluate this question (Table 1). The results of these studies will further inform whether the combination of thoracic SBRT and ICPIs can be delivered safely and effectively.
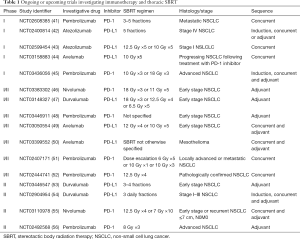
Full table
SBRT boost
The use of SBRT as a boost for residual disease following definitive chemoradiotherapy (CRT) is a relatively novel indication. Any tissue within the SBRT boost PTV would receive an elevated cumulative radiation dose, potentially increasing the risk of severe toxicity.
Two retrospective trials have evaluated the use of SBRT for dose escalation following definitive CRT. Karam et al. treated 16 patients with CRT to a median dose of 50.4 Gy followed by an SBRT boost using a robotic stereotactic system (Cyberknife®) to an average additional dose of 25 Gy (range, 20–30 Gy) in 5 fractions. The range of median time interval between IMRT and SBRT was 20 days with a range of 7–97 days. Their boost volume included any residual tumor and any clinically involved lymph nodes. Four patients (25%) reported grade 2 acute pneumonitis, only one of whom required hospitalization. One patient who received a cumulative dose of 90 Gy developed a dry cough which progressed to hemoptysis and a pneumothorax. The range of cumulative biologically equivalent dose (BED10) received was 81.1–120 Gy, with a median of 97.0 Gy. The treatment was well tolerated; there were no grade 3 or worse toxicities or treatment-related deaths reported (57).
An Italian group retrospectively evaluated 17 patients who received conventional radiation therapy to 50–60 Gy in 20–30 fractions and received further SBRT to in-field centrally located recurrences or persistent disease. Post-treatment 18FDG-PET avidity was used to determine the GTV and was boosted with an additional 30 Gy in 5–6 fractions. Grade 3 pneumonitis was seen in 4 patients (23%). Two fatalities were reported; one patient developed grade 5 pneumonitis 4 months following SBRT and another patient, who had persistent disease involving the hilum, developed grade 5 hemoptysis 2 months after SBRT (58).
Investigators at the University of Kentucky conducted a prospective, single-institution Phase I study evaluating the feasibility of SBRT boost to residual primary disease following CRT. Patients were initially treated to an additional dose of 20 Gy in 2 fractions. After enrolling 16 patients, however, 2 patients with fatal grade 5 hemoptysis were identified, both with large cavitary recurrences involving the hilum. Subsequently, patients with centrally located tumors were treated to a dose of 19.5 Gy in 3 fractions (59).
Feddock et al. published a post-hoc analysis of a subset of these patients with central tumors, including the two patients with grade 5 hemoptysis. Analysis of these patients included distance of the PTV to the pulmonary artery, maximum dose to the pulmonary artery, maximum dose to the bronchial tree and other dosimetric variables, none of which correlated with risk of fatal pulmonary hemorrhage. Local recurrence was the only factor associated with a significantly increased risk of hemorrhage. The authors concluded that SBRT boost can be done safely, although with careful limitation of the central structures. Specifically, for a patient who has received 60–66 Gy from CRT, they recommend limiting dose to both the pulmonary artery and the bronchovascular tree wall to <700 cGy ×3 or <900 cGy ×2 fractions for additional SBRT boost (60).
Other patients treated at the University of Kentucky tolerated treatment well. The initial report of 35 patients with a median follow up of 13 months reported 4 patients with acute grade 3 pneumonitis and 1 patient with late grade 3 pneumonitis. There were no instances of grade 4–5 pneumonitis. One patient developed grade 2 acute esophagitis and 2 patients had a bronchial stricture that did not require endobronchial intervention (59). An updated analysis with median follow up of 25.2 months reported an association between higher mean lung dose during CRT and grade 3 pneumonitis; no additional late toxicity was reported (61).
Another Phase I dose escalation study of SBRT in 2 fractions following 50.4 Gy CRT aimed to identify the maximum tolerated dose (MTD) for SBRT boost. The GTV included residual primary and nodal disease. The maximum tolerated SBRT dose was 14 Gy ×2 without any grade ≥3 toxicities within 4 weeks of treatment. There was one fatal bronchopulmonary hemorrhage 13 months following treatment which was reported as a grade 5 event. Further analysis revealed that the dose to the proximal bronchial tree (PBT) was significantly higher than that of other patients in the cohort, and the dose received by 4 cc of the PBT (D4cc) was significantly associated with pulmonary hemorrhage. The authors concluded that SBRT boost is relatively safe but recommended strict dose-volume constraints to the PBT (D1cc <20 Gy or D4cc <15 Gy), in addition to consideration of a 9 Gy ×3 fraction regimen rather than 12 Gy ×2 fractions for SBRT boost (62).
Higgins et al. from Emory University published the results of their Phase I dose escalation trial which aimed to identify the MTD. Fifteen patients were treated to an initial CRT dose of 44 Gy followed by SBRT boost to any residual primary and nodal disease to an additional dose of 9 Gy ×2, 10 Gy ×2, 6 Gy ×5 or 7 Gy ×5. DLT was defined as any grade ≥3 adverse event. The MTD was calculated at 6 Gy ×5 with one DLT in the form of a tracheoesophageal fistula, from which the patient died following repair. The previous dose level, 10 Gy ×2, had no associated DLTs. As such, the authors suggested 10 Gy ×2 as a reasonable dose for SBRT boost (63).
Although the literature available is limited by its largely retrospective nature, SBRT boost appears to be well tolerated in the peripheral lung, but needs to be performed carefully when the boost volume contains the hilum or central structures. Reports of dose-related fatal toxicities have included hemorrhage, tracheoesophageal fistula and pneumonitis. The difference in toxicity incidence and grade between the many retrospective studies suggests that patient selection, technique and experience may be significant factors in the incidence of adverse events. It is especially relevant to note that the SBRT boost is typically performed after the conclusion of concurrent chemoradiation to a definitive or near definitive dose when normal tissues are presumably the most sensitive to further injury. Ongoing clinical trials investigating the use of SBRT boost including another dose-escalation trial (NCT01345851) (64) and a phase II trial at Ohio State University investigating SBRT boost prior to definitive CRT (NCT02262325) (65) will hopefully provide more information regarding the efficacy and toxicity of this technique.
Conclusions
Stereotactic body radiation therapy is now considered a standard treatment option for early stage NSCLC and oligometastatic disease. Risk factors for development of several treatment-related toxicities, such as chest wall pain and rib fracture, have been elucidated. However, care must still be taken when delivering SBRT to patients with central tumors and in those receiving immune checkpoint inhibitors. Ongoing investigations will provide additional guidance for practitioners facing these challenging clinical scenarios.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kang KH, Okoye CC, Patel RB, et al. Complications from Stereotactic Body Radiotherapy for Lung Cancer. Cancers 2015;7:981-1004. [Crossref] [PubMed]
- De Rose F, Franceschini D, Reggiori G, et al. Organs at risk in lung SBRT. Phys Med 2017;44:131-8. [Crossref] [PubMed]
- Murray L, Karakaya E, Hinsley S, et al. Lung stereotactic ablative radiotherapy (SABR): dosimetric considerations for chest wall toxicity. Br J Radiol 2016;89:20150628. [Crossref] [PubMed]
- Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys 2011;80:692-7. [Crossref] [PubMed]
- Welsh J, Thomas J, Shah D, et al. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2011;81:91-6. [Crossref] [PubMed]
- Stephans KL, Djemil T, Tendulkar RD, et al. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT). Int J Radiat Oncol Biol Phys 2012;82:974-80. [Crossref] [PubMed]
- Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving > 30 gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:796-801. [Crossref] [PubMed]
- Park Y, Kim HJ, Chang AR. Predictors of chest wall toxicity after stereotactic ablative radiotherapy using real-time tumor tracking for lung tumors. Radiat Oncol 2017;12:66. [Crossref] [PubMed]
- Thibault I, Chiang A, Erler D, et al. Predictors of Chest Wall Toxicity after Lung Stereotactic Ablative Radiotherapy. Clin Oncol (R Coll Radiol) 2016;28:28-35. [Crossref] [PubMed]
- Taremi M, Hope A, Lindsay P, et al. Predictors of Radiotherapy Induced Bone Injury (RIBI) after stereotactic lung radiotherapy. Radiat Oncol 2012;7:159. [Crossref] [PubMed]
- Stam B, van der Bijl E, Peulen H, et al. Dose-effect analysis of radiation induced rib fractures after thoracic SBRT. Radiother Oncol 2017;123:176-81. [Crossref] [PubMed]
- Mutter RW, Liu F, Abreu A, et al. Dose-volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int J Radiat Oncol Biol Phys 2012;82:1783-90. [Crossref] [PubMed]
- Chao HH, Valdes G, Luna JM, et al. Exploratory analysis using machine learning to predict for chest wall pain in patients with stage I non-small-cell lung cancer treated with stereotactic body radiation therapy. J Appl Clin Med Phys 2018;19:539-46. [Crossref] [PubMed]
- Pettersson N, Nyman J, Johansson K-A. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: A dose- and volume-response analysis. Radiother Oncol 2009;91:360-8. [Crossref] [PubMed]
- Asai K, Shioyama Y, Nakamura K, et al. Radiation-Induced Rib Fractures After Hypofractionated Stereotactic Body Radiation Therapy: Risk Factors and Dose-Volume Relationship. Int J Radiat Oncol Biol Phys 2012;84:768-73. [Crossref] [PubMed]
- Okoukoni C, Lynch SK, McTyre ER, et al. A cortical thickness and radiation dose mapping approach identifies early thinning of ribs after stereotactic body radiation therapy. Radiother Oncol 2016;119:449-53. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with "Ultracentral" Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1081-9. [Crossref] [PubMed]
- Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015;89:50-6. [Crossref] [PubMed]
- Bradley JD, Gao F, Parikh PJ, et al. Prospective Phase 2 Clinical Trial of Radiation Dose-Escalated Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Lung Cancer: An Institutional Trial. Int J Radiat Oncol Biol Phys 2015;93:S101-S.
- Bezjak A, Paulus R, Gaspar LE, et al. Primary Study Endpoint Analysis for NRG Oncology/RTOG 0813 Trial of Stereotactic Body Radiation Therapy (SBRT) for Centrally Located Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2016;94:5-6. [Crossref]
- Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiat Oncol Evidence-Based Guideline. J Clin Oncol 2018;36:710-9. [Crossref] [PubMed]
- Stam B, Grills IS, Kwint M, et al. SBRT for Central Tumors in Early Stage NSCLC Patients. Int J Radiat Oncol Biol Phys 2017;99:S17. [Crossref]
- Modh A, Rimner A, Williams E, et al. Local Control and Toxicity in a Large Cohort of Central Lung Tumors Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2014;90:1168-76. [Crossref] [PubMed]
- Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol 2016;6:E27-33. [Crossref] [PubMed]
- Horne ZD, Richman AH, Dohopolski MJ, et al. Stereotactic body radiation therapy for isolated hilar and mediastinal non-small cell lung cancers. Lung Cancer 2018;115:1-4. [Crossref] [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- SUNSET: SBRT for Ultra-central NSCLC- a Safety and Efficacy Trial. Available online: https://ClinicalTrials.gov/show/NCT03306680, Lawson Health Research Institute. Accessed 3/14/2018.
- Hematology/Oncology (Cancer) Approvals & Safety Notifications. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Accessed 3/14/2018.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (version 3.2018). Available online: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375-91. [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref]
- Bhalla N, Brooker R, Brada M. Combining immunotherapy and radiotherapy in lung cancer. J Thorac Dis 2018;10:S1447-60. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Ahmed KA, Grass GD, Creelan B, et al. Tolerability and Safety of Thoracic Radiation and Immune Checkpoint Inhibitors Among Patients with Lung Cancer. Int J Radiat Oncol Biol Phys 2017;98:224. [Crossref]
- Study of PD1 Blockade by Pembrolizumab With Stereotactic Body Radiotherapy in Advanced Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02608385, University of Chicago. Accessed 3/14/2018.
- MPDL3280A and Stereotactic Ablative Radiotherapy in Patients With Non-small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT02400814, University of California, Davis National Cancer Institute Genentech, Inc. Accessed 3/14/2018.
- Atezolizumab and Stereotactic Body Radiation Therapy in Treating Patients With Non-small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT02599454, University of California, Davis Genentech, Inc. Accessed 3/14/2018.
- Avelumab and Stereotactic Ablative Radiotherapy in Non-responding and Progressing NSCLC Patients. Available online: https://ClinicalTrials.gov/show/NCT03158883, University of California, Davis EMD Serono. 2017. Accessed 3/14/2018.
- PembRolIzuMab and Stereotactic Body Radiotherapy In Metastatic Non-small-cell lunG Cancer Patients. Available online: https://ClinicalTrials.gov/show/NCT03436056, Royal Marsden NHS Foundation Trust Merck Sharp Dohme Corp. Accessed 3/14/2018.
- SBRT With Immunotherapy in Early Stage Non-small Cell Lung Cancer: Tolerability and Lung Effects. Available online: https://ClinicalTrials.gov/show/NCT03383302, Royal Marsden NHS Foundation Trust Bristol-Myers Squibb. Accessed 3/14/2018.
- Astra Zeneca (Immuno Stereotactic Ablative Body Radiotherapy) ISABR Study: Randomized Phase I/II Study of Stereotactic Body Radiotherapy. Available online: https://ClinicalTrials.gov/show/NCT03148327, Jonsson Comprehensive Cancer Center AstraZeneca National Cancer Institute. Accessed 3/14/2018.
- Combining SBRT and Immunotherapy in Early Stage NSCLC Patients Planned for Surgery. Available online: https://ClinicalTrials.gov/show/NCT03446911, VU University Medical Center. Accessed 3/14/2018.
- Stereotactic Body Radiation Therapy (SBRT) Combined With Avelumab (Anti-PD-L1) for Management of Early Stage Non-Small Cell Lung Cancer (NSCLC). Available online: https://ClinicalTrials.gov/show/NCT03050554, Andrew Sharabi Pfizer University of California, San Diego. Accessed 3/14/2018.
- Stereotactic Body Radiation Therapy and Avelumab Immunotherapy for Treatment of Malignant Mesothelioma. Available online: https://ClinicalTrials.gov/show/NCT03399552, Memorial Sloan Kettering Cancer Center Pfizer. Accessed 3/14/2018.
- Evaluating the Combination of MK-3475 and Sterotactic Body Radiotherapy in Patients With Metastatic Melanoma or NSCLC. Available online: https://ClinicalTrials.gov/show/NCT02407171, Yale University. Accessed 3/14/2018.
- MK-3475 and Stereotactic Body Radiation Therapy (SBRT) in Patients With Non-Small Cell Lung Cancer (NSCLC). Available online: https://ClinicalTrials.gov/show/NCT02444741, M.D. Anderson Cancer Center Merck Sharp Dohme Corp. Accessed 3/14/2018.
- Ablative STEreotactic RadiOtherapy wIth Durvalumab (MEDI4736). Vastra Gotaland Region AstraZeneca. Available online: https://ClinicalTrials.gov/show/NCT03446547. Accessed 3/14/2018.
- Durvalumab (MEDI4736) With or Without SBRT in Clinical Stage I, II and IIIA Non-small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT02904954, Weill Medical College of Cornell University AstraZeneca.
- Clinical Trials Comparing Immunotherapy Plus Stereotactic Ablative Radiotherapy (I-SABR) Versus SABR Alone for Stage I, Selected Stage IIa or Isolated Lung Parenchymal Recurrent Non-Small Cell Lung Cancer: I-SABR. Available online: https://ClinicalTrials.gov/show/NCT03110978, M.D. Anderson Cancer Center Bristol-Myers Squibb. Accessed 3/14/2018.
- Pembrolizumab After SBRT Versus Pembrolizumab Alone in Advanced NSCLC. Available online: https://ClinicalTrials.gov/show/NCT02492568, The Netherlands Cancer Institute Merck Sharp Dohme Corp. Accessed 3/14/2018.
- Karam SD, Horne ZD, Hong RL, et al. Dose escalation with stereotactic body radiation therapy boost for locally advanced non-small cell lung cancer. Radiat Oncol 2013;8:179. [Crossref] [PubMed]
- Trovo M, Minatel E, Durofil E, et al. Stereotactic Body Radiation Therapy for Re-irradiation of Persistent or Recurrent Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2014;88:1114-9. [Crossref] [PubMed]
- Feddock J, Arnold SM, Shelton BJ, et al. Stereotactic Body Radiation Therapy Can Be Used Safely to Boost Residual Disease in Locally Advanced Non-Small Cell Lung Cancer: A Prospective Study. Int J Radiat Oncol Biol Phys 2013;85:1325-31. [Crossref] [PubMed]
- Feddock J, Cleary R, Arnold S, et al. Risk for fatal pulmonary hemorrhage does not appear to be increased following dose escalation using stereotactic body radiotherapy (SBRT) in locally advanced non-small cell lung cancer (NSCLC). J Radiosurg SBRT 2013;2:235-42. [PubMed]
- Kumar S, Feddock J, Li X, et al. Update of a Prospective Study of Stereotactic Body Radiation Therapy for Post-Chemoradiation Residual Disease in Stage II/III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:652-9. [Crossref] [PubMed]
- Hepel JT, Leonard KL, Safran H, et al. Stereotactic Body Radiation Therapy Boost After Concurrent Chemoradiation for Locally Advanced Non-Small Cell Lung Cancer: A Phase 1 Dose Escalation Study. Int J Radiat Oncol Biol Phys 2016;96:1021-7. [Crossref] [PubMed]
- Higgins KA, Pillai RN, Chen Z, et al. Concomitant Chemotherapy and Radiotherapy with SBRT Boost for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase I Study. J Thorac Oncol 2017;12:1687-95. [Crossref] [PubMed]
- Image-Guided Hypofractionated Radiation Therapy With Stereotactic Body Radiation Therapy Boost and Combination Chemotherapy in Treating Patients With Stage II-III Non-Small Cell Lung Cancer That Cannot Be Removed By Surgery. Available online: https://ClinicalTrials.gov/show/NCT01345851. Accessed 3/14/18.
- Hypofractionated Boost Before Chemoradiation for Patients With Stage II-III Non-small Cell Lung Cancer Unsuitable for Surgery. Available online: https://ClinicalTrials.gov/show/NCT02262325, Ohio State University Comprehensive Cancer Center. Accessed 3/14/18.


