Italian multicenter survey to evaluate the opinion of patients and their reference clinicians on the “tolerance” to targeted therapies already available for non-small cell lung cancer treatment in daily clinical practice
Introduction
The knowledge of molecular mechanisms of cancer pathogenesis has led to the discovery of pathways directly involved in cell transformation. In patients’ subgroups, malignancies can result from genetic alterations in a single gene. The cancer becomes dependent upon, or “addicted” to, signaling from a specific transcribed protein which often is a tyrosine kinase receptor. In advanced non-small cell lung cancer (NSCLC), the epidermal growth factor receptor (EGFR) and the anaplastic lymphoma kinase (ALK) are proteins for which specific inhibitors are currently available in the clinical practice. About 10-15% of Caucasians are affected by advanced NSCLC “addicted” to activating mutations of EGFR and receive greater benefit from first-line EGFR-tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib and afatinib (1). Erlotinib is currently licensed also for unselected pretreated NSCLC patients on the basis of survival benefit reported compared with placebo in the BR.21 trial (2). In another 2-5% of patients, NSCLC is “addicted” to ALK-translocations and crizotinib, an ALK-TKI, is currently the standard second-line therapy for this subset of patients (3) (at least in Italy). All these TKIs are small molecules administered orally daily and are used according to the “personalized medicine” model, for which every single tumor might potentially benefit from a specific individual treatment, reaching unprecedented impressive outcomes in NSCLC (1,3). Among the many anti-angiogenic compounds that have been tested in advanced NSCLC, only bevacizumab has been approved in first line treatment in association with carboplatin-paclitaxel or cisplatin-gemcitabine. To date, no specific target has been identified to select the population receiving greater benefits from this treatment, as in fact the choice is currently based on clinical, radiologic and histological characteristics.
Despite the high selectivity of TKIs and the growing amount of data coming from clinical trials, in daily clinical practice several previously unknown and sometimes unpredictable adverse events are emerging directly related with targets also present in normal tissues, and which are inhibited or modulated by the specific drug. The main organs and systems involved are: the skin and mucous membranes, the gastrointestinal tract, the cardiovascular, respiratory and neurologic systems (1-3). Nevertheless, treating clinicians must be challenged to recognize and measure these “new” adverse events and understand how to manage them because, even if in the majority of cases they are of mild-moderate grade and reversible on treatment cessation, they could deeply influence patients’ quality-of-life (QoL).
Several reports underlined a strong discrepancy between patients’ self-assessment and investigators’ evaluation of the presence and grade of symptoms related to the treatment (4). Based on these considerations, we investigated the toxicity profile and their impact on patients’ daily life when treated with targeted therapies, measuring the patient point of view and comparing this with that of the referring physician. The aim was to highlight any discrepancy and evaluate how this should be corrected in order to improve management.
Methods
Between October 2013 and April 2014, 133 patients with advanced NSCLC (IIIB and IV stages, according to the UICC TNM 7th edition), treated with targeted therapies at any lines of treatment within seven Oncologic Institutions involved in this Italian multicentre survey, were consecutively and prospectively enrolled. They were assessed for toxicities and impact on QoL with specific monthly questionnaires. The questionnaire and the project were designed and supported by WALCE Onlus (Women Against Lung Cancer in Europe), a European association devoted to lung cancer patients and their families in terms of information, support and primary prevention (5). Achievement of main inclusion criteria required a treatment with an approved targeted therapy (allowing also the enrollment of patients already included in clinical trials), at least 15 days of therapy since the beginning of the biological treatment (T0), patient age ≥18 years, a life expectancy ≥6 months (according to clinical judgment of the referring physician) and adequate verbal comprehension skills for completing the questionnaires provided. No limitations as regards type of targeted therapy employed, number of cycles previously administered and kind of preceding oncologic treatments were imposed.
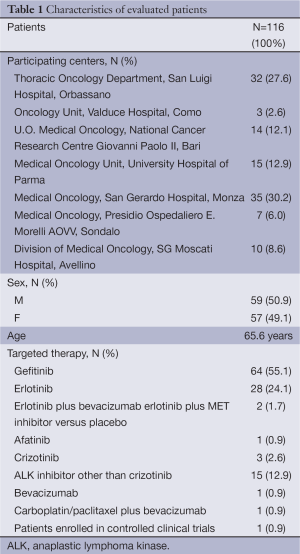
Among the 133 patients recruited, 116 were considered suitable for final analysis because they attended at least three consecutive monthly evaluations (T0, T1, T2) to reach a meaningful outcome. At every checkpoint, patients and referring clinicians received a specific questionnaire (and relative explanation letter) for the assessment of skin toxicity, oral mucositis, fatigue, visual disorders, nausea, diarrhea and hypertension potentially related to targeted therapies observed during the treatment and the impact of these toxicities on QoL. The remaining 17 patients, considered not suitable for final evaluation, had disease progression that required a therapeutic shift (14 cases) or a worsening of clinical conditions that did not allow other oncologic treatments, but only best supportive care (3 cases). All patients fulfilled all questionnaires at all checkpoints immediately before, while their referring clinicians after the scheduled visit. Table 1 summarizes the participating centers, main characteristics of the 116 evaluable patients and targeted therapies.
Full table
The survey required completion of both questionnaires (physicians and patients), basing adverse event evaluation on the CTCAE version 4.0 (6). The points investigated were chosen according to the major reported toxicities in association with approved targeted drugs in advanced NSCLC, and are listed in Table 2.

Full table
Statistical analysis of collected qualitative data was performed using Fisher’s exact test. Two-tailed P values were used, and results were considered significant at P values <0.05.
Results (Figures 1-3)
Among the 116 patients included in the final analysis, 98 (84.5%) were treated with an EGFR-TKI (used prevalently alone, but in combination with an antiangiogenetic drug in two cases, and with a MET-inhibitor in one case, since these three patients were included in controlled clinical trials), 16 patients (13.8%) with an ALK inhibitor, 1 patient with bevacizumab plus platinum-based chemotherapy and 2 patients (1.7%) with a single antiangiogenic agent, without no addition of biological drugs (Table 1). Median duration of treatment was 13.4 months (range, 2.7-76.1 months).
At baseline (T0), among the 232 questionnaires evaluated (116 from patients and 116 from clinicians, respectively) a statistically significant difference in terms of perception of any-type and any-grade targeted therapies-related toxicities was described (two tailed P value =0.0001). Only few cases of any-type grade 3 and 4 toxicities were described, according to the good treatment safety profile. Similar results were observed for T1 and T2 (two tailed P value =0.0001 and 0.0001, respectively).
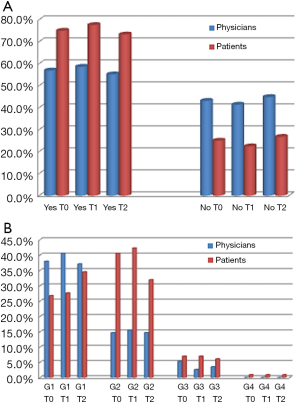
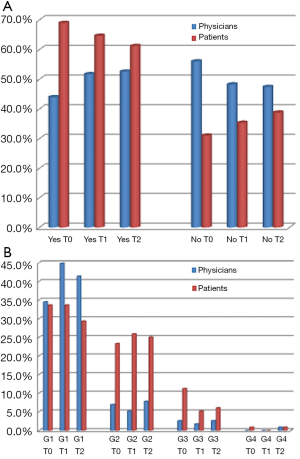
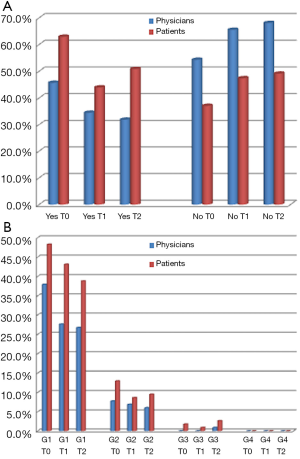
Relevant data in terms of different perception of toxicities between patients and referring clinicians were observed at every checkpoint (T0, T1, T2) especially for skin toxicity, fatigue and diarrhea (Figure 1A, Figure 2A, Figure 3A, respectively). About 75.0%, 77.6% and 73.3% of patients, at T0, T1 and T2, respectively, declared to have experienced any grade skin toxicity during the preceding weeks of treatment, compared to 56.9%, 58.6% and 55.2%, respectively, according to clinicians’ evaluation. Two-tailed Fisher’s exact P value was 0.0054, 0.003 and 0.006, respectively. Analysis of skin toxicity grades revealed a lower perception of grade 1 toxicitiy by patients, when compared to their referring clinicians, and a higher perception of grade 2 toxicities (Figure 1B). Similar data were observed for fatigue: evaluation of patients’ questionnaires revealed 69.0%, 64.6% and 61.2% of targeted therapies-related fatigue, at T0, T1 and T2, respectively, compared with 44.0%, 51.7% and 52.6%, respectively, according to clinicians’ evaluation. Two-tailed Fisher’s exact P value was 0.0002, 0.0621 and 0.2327, respectively. Similarly to skin toxicity grades, at every checkpoint (T0, T1, T2) patients’ evaluation of fatigue, compared to clinicians’, resulted lower for grade 1, higher for grade 2, minimal for grade 3 and 4 (Figure 2B). Patients’ questionnaires revealed 62.9%, 44.0% and 50.9% of targeted therapies-related diarrhea at T0, T1 and T2, respectively, compared with 45.7%, 34.5% and 31.9%, respectively, from clinicians’ questionnaires. Two-tailed Fisher’s exact P value was 0.0121, 0.0079 and 0.0050, respectively. Overall, patients’ perception of any-grade diarrhea resulted higher when compared with clinicians’ evaluation (Figure 3B).
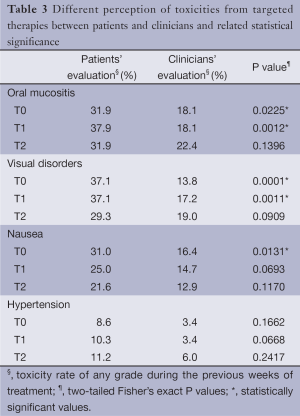
A similar trend was observed for the other four items evaluated, summarized in Table 3. For hypertension in particular, 18.1%, 13.8% and 11.2% of patients, at T0, T1 and T2 respectively, did not know their blood pressure value.
Full table
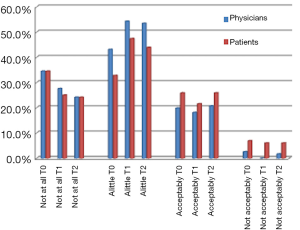
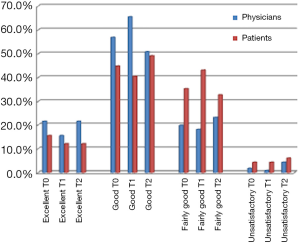
Targeted therapies were in general assessed as well tolerated by both patients and referring clinicians. The assessment of QoL also revealed discordant data, showing for patients, when compared with clinicians, a lower perception of targeted therapies-related influence on daily life when deemed slightly influenced, but a higher perception when considered not interfering at all or acceptably influenced with daily activities and relationships (Figure 4). At T0, QoL was deemed excellent, good, fairly good and unsatisfactory by 15.5%, 44.8%, 35.4% and 4.3% of patients, respectively, compared with 21.5%, 56.9%, 19.9% and 1.7% of clinicians (Figure 5). Similar results were observed at T1 and T2.
Discussion
The development of a large number of targeted therapies for NSCLC treatment in the past decade has led to the possibility to better personalize the therapeutic options for patients with specific clinical characteristics and molecular features. However, this has also led to the emerging of related toxicities, whose management and impact on QoL is frequently challenging. Difficulties in the management of such toxicities often come from the accelerated shift from clinical trial to clinical practice settings of these new classes of drugs, and from the onset of previously unexpected adverse events. Despite the fairly positive toxicity profile of targeted therapies, some patients are extremely sensitive to these drugs and can develop severe toxicities which can impact their daily life and influence QoL. Moreover, discrepancy between patients’ and clinicians’ perception of targeted therapies-related toxicities makes the daily clinical effort that these patients require even more difficult.
Analysis of the results of this Italian multicenter survey emphasizes a probably general underestimation of toxicities by clinicians when compared to patients’ perception. This trend was observed at baseline (T0), but also at every subsequent checkpoints (T1, T2), probably due to the attitude of clinicians in evaluating the toxicities from targeted therapies over time and, simultaneously, to the very low cumulative effect of such toxicities according to patients’ evaluation.
Discrepancy between patients and clinicians evaluation of toxicities seems to be greater for adverse events more strongly associated with daily life and QoL, such as skin toxicity, fatigue and diarrhea. This was observed for CTCAE version 4.0 grade 2 or higher skin toxicity, fatigue, and for any-grade diarrhea (Figure 1B, Figure 2B, Figure 3B).
Data regarding influence of targeted therapies on daily life strongly reflect literature data of good tolerance for long-term biological treatments in NSCLC patients (6-8).
As for Quinten et al. (9), in this survey patients-reported scores differed from clinicians-reported scores. In this publication, baseline data regarding six cancer symptoms (pain, fatigue, vomiting, nausea, diarrhea, and constipation) from a total of 2,279 cancer patients from 14 closed EORTC randomized controlled trials were analyzed. In each trial, selected for retrospective pooled analysis, both clinicians and patients’ symptoms scoring were reported. In this study, different opinions were reported between patients and clinicians, similarly to what we described in our survey, but the Authors reported also a prognostic impact on survival. Cox models of overall survival that took into account both patients and clinicians’ scores gained more predictive accuracy than models that took into account clinicians’ scores alone for each of four symptoms: fatigue, vomiting, nausea, and constipation (9). This stresses the need to have patients’ reports together with referring clinicians’ clinical outcomes.
The impact on daily activities and relationships was globally assessed as not to interfere at all by a consistent number of patients, similarly to the clinicians’ perception, underlining the great clinical benefit of this kind of treatment, besides the possibility of disease control. On the contrary, when impact on daily life was considered slightly influenced by targeted therapies, the assessment of patients resulted lower in comparison to the referring clinicians, probably due to the impact of invalidating toxicities on their relationships and activities (Figure 4).
At every checkpoint, most patients reported QoL as good (44.8% at T0), although their evaluation was globally lower when compared to clinicians’ perception (56.9% at the same checkpoint), probably because QoL was strongly influenced by symptoms and distress related to the disease (Figure 5).
One weakness of this survey could be related to the fact that few patients underwent therapy with bevacizumab and this might limit the ability to interpret the data for this subgroup, although the percentage is quite consistent with the use of this drug in this context in Italy.
One strength is the design of the survey, prospectively collecting information specifically in patients with advanced NSCLC treated with already approved targeted drugs.
The poor perception of clinicians of the most invalidating toxicities according to patients’ evaluation should be a crucial result from which to start thinking about a new approach for a better evaluation of the impact of targeted-therapy-related toxicities on patients’ general conditions, relationships and QoL. The questionnaires employed in this survey were extremely simple to understand and fill in by both patients and clinicians. A few minutes to answer to the questions proposed can directly address very important aspects for patients’ health and psychological well-being. Moreover, a better understanding by clinicians of the clinical and psychological distress related to a targeted treatment regimen could enhance the relationship between patients and his/her referring clinicians, but also improve the therapeutic intervention in case of toxicities.
Conclusions
Despite this Italian multicenter survey was the first experience in evaluating toxicities of targeted therapies with a new specific monthly questionnaire, and the need of a larger number of evaluated patients to confirm the collected data, the results from this first analysis are promising. The questionnaires proposed could be an effective tool to understand the approach to a better management of targeted-therapy-related toxicities, and to avoid the current discrepancy between patients and clinicians perception of adverse events and their impact on QoL.
Acknowledgements
Authors’ contribution: WALCE, www.womenagainstlungcancer.eu, (with clinicians and advocates contribution) designed the overall structure of this Italian multicenter survey with the contribution of all the other authors who co-wrote and finally approved the manuscript for publication. All authors were involved in the collection of data, subsequently analyzed by first authors, Enrica Capelletto and Simonetta Grazia Rapetti.
All authors of this manuscript have directly participated in its planning and execution. A special thank you to the patients who actively contributed to the collection of this important data.
Disclosure: The authors declare no conflict of interest.
References
- Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J Clin Oncol 2013;31:1081-8. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Solomon B, Wilner KD, Shaw AT. Current status of targeted therapy for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Clin Pharmacol Ther 2014;95:15-23. [PubMed]
- Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst 2011;103:1808-10. [PubMed]
- Available online: www.womenagainstlungcancer.eu
- Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7. pdf. Published on 2009, modified in June 2010
- Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin 2013;63:249-79. [PubMed]
- Ricciardi S, Tomao S, de Marinis F. Toxicity of targeted therapy in non-small-cell lung cancer management. Clin Lung Cancer 2009;10:28-35. [PubMed]
- Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 2011;103:1851-8. [PubMed]