
Update on screening for lung cancer
Lung cancer is the leading cause of cancer-related deaths in most developed countries, and is responsible for enormous financial costs and physical suffering (1). Treatment is more effective, and cure is more common when it is detected at an early stage. These facts make lung cancer an obvious target for population-based public health interventions, including screening. However, until 2011 no screening modality had demonstrated the potential to reduce the burden of lung cancer morality. Multiple large-scale clinical trials in the 1970s employed varied combinations of chest X-ray (CXR) with or without sputum cytology to screen for lung cancer. While all if these trials showed that such screening could identify more cancers, all failed to demonstrate a reduction in lung cancer mortality (2-6), the only valid metric for the effectiveness of a screening program. This changed with the release of the results of the National Lung Screening Trial (NLST) in 2011. The NLST enrolled over 53,000 individuals between the ages of 55 and 75, who had heavy current or recent tobacco use history. Study participants received three rounds of screening either with low-dose CT, or standard chest radiography. The study found a 20% reduction in lung cancer specific mortality in those screened annually for three rounds with low-dose CT scans (7). This started a new era in which lung cancer screening is now increasingly recommended by nearly every professional organization (8-13) as well as by the United States Preventive Services Task Force (USPSTF) (14). More recently, the results of a second lung cancer screening trial conducted in European countries further confirmed the potential for screening with CT scans to reduce lung cancer mortality (15).
The importance of “early detection” has become almost mythically engrained in our understanding of disease. Yet even among physician experts, understanding the biases involved in judging the effectiveness of screening interventions and the risks/benefits of cancer screening is poor (16). While typically necessary for an effective cancer screening program “early detection”, is not sufficient to demonstrate the effectiveness of such an intervention. Only a reduction in cancer specific mortality can justify the institution of a population-based screening program. A survey given to physicians attending a national conference showed that most incorrectly identified a screening intervention that lead to “prolonged survival” as a valid metric of screening efficacy (16). This gap in knowledge has important consequences when physicians are involved in counseling their patients about cancer screening. In the U.S. the Center for Medicare Services (CMS) required “Shared Decision Making” for reimbursement when issuing a coverage decision for lung cancer screening. Shared decision making requires, at a minimum, that the provider be able to recognize and convey the benefits and risks involved in any given choice about health care in terms understandable to the patient. If physicians and other providers do not understand these risks and benefits, they cannot effectively lead a discussion with patients who may be less informed, even if they have the time to do so (17). Resolving this paradox and helping physicians identify both the benefits and potential harms of screening is part of the purpose of this manuscript. This update will overview the data that support lung cancer screening as a public health intervention, suggest who should be screened, who should not be screened, how screening should be done, and point out important yet overlooked means to ensure that the benefits of a mass screening program truly outweigh any harms.
Who should be screened?
In the U.S., guidance on who should be offered lung cancer screening has been based on modeling of the results of the NLST on the basis of the U.S. population of smokers (14), or on “consensus” expert opinions (18). The NLST was a randomized controlled trial of over 53,000 subjects between the ages of 55, and 74, eligible on the basis of current, or former (within 15 years) heavy smoking (greater than 30 pack-years), at the time of initial screening. A major impetus for the NLST were results from nonrandomized trials beginning in the 1990s (19,20) showing the feasibility of CT scans for screening for cancer. These trials showed that lung cancer screening could be performed with low-dose CT scans, and offered the possibility of detection of very early stage cancers, with an acceptable rate of false positives and of invasive procedures done for otherwise benign disease. Enthusiastic recommendations for lung cancer screening based solely on “prolonged survival” are misguided though. Only the demonstration of reduced lung cancer specific mortality can justify recommendations for mass screening. The NLST was the first randomized trial of a lung cancer screening test to demonstrate the potential for reducing lung cancer mortality. After 9 years of follow-up, among the 53,000 participants, there were 346 lung cancer deaths in the low-dose CT group and 425 deaths in the control (CXR) group, representing a relative reduction in mortality of 20%. Screening recommendations by the USPSTF are based on modeling of the results of the NLST in the population of U.S. smokers eligible for screening. The importance of this modeling is that the eligible population of smokers in the U.S. is different from the subjects in the NLST in important ways. The U.S. population eligible for CT lung cancer screening is older, sicker and has less reliable access to health care in comparison to participants in the NLST (21). While these differences are associated with a higher risk of cancer, they are also associated with worse outcomes from diagnostic, and treatment interventions. As a result, the 20% reduction in lung cancer mortality seen in the NLST cannot be expected to fully translate to practice. Real world experience already suggests much higher complication rates in clinical practice than were reported in the NLST (22).
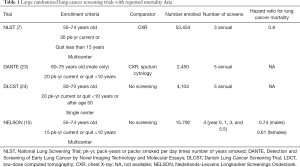
More recently, even more encouraging results from a randomized trial of lung cancer screening in the Dutch-Belgian Lung Cancer Screening Trial (Nederlands-Leuvens Longkanker Screenings Onderzoek; NELSON) demonstrated a larger mortality benefit when individuals were screened with CT scans at less frequent intervals and in which participants may have had a lower risk as measured by age and pack-years of tobacco exposure (15). Throughout Europe, and parts of Asia, health systems are beginning to institute mass lung cancer screening programs based on these, and other trials (Table 1). Of all the stakeholder organizations in the U.S., only the American Academy of Family Practice has withheld a formal recommendation in favor of lung cancer screening (25), with an active debate among the membership (26,27).
Full table
There is no consensus from one program to the next on the exact population for whom screening should be offered. However, there is universal consensus that screening should be offered to people with apparent high risk for lung cancer, and this risk is almost exclusively defined on the basis of tobacco use history. While this is, at best, an imperfect criterion for selection of screening eligible individuals, there is currently no alternative selection criteria which have been shown to maximize the benefits of screening (reduced mortality), without increasing the potential harm (false positives, and over diagnosis, and invasive procedures on people with benign disease). Current recommendations in the U.S. are to offer screening to otherwise healthy individuals between the ages of 55 and 77, with current, or recent heavy smoking history. Other risk factors which have been consistently shown to increase the risk for lung cancer include a diagnosis of chronic obstructive pulmonary disease (COPD), a family history of first-degree relatives with lung cancer, and a prior diagnosis of cancer, excluding non-melanoma skin cancer (28-30). Many individuals rightly ask about asbestos exposure, secondhand smoke exposure, or radon exposure. While these are well-established epidemiologic risk factors for lung cancer, the ability to incorporate these into existing risk models is limited by the inability to quantify, and explicitly define such exposures. Screening criteria for some of the largest lung cancer screening trials are shown in Table 1, as well as the mortality benefit (if any) observed for screening in that population.
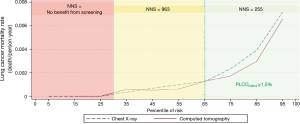
What is the ideal level of risk at which screening should be considered? This has not been explicitly considered in the context of a clinical trial, but investigators have used modeling to pose such a question. Tammemägi et al. tried to determine a level of lung cancer risk at which mortality from lung cancer was consistently improved by screening (31). Applying the PLCOm2012 model, a model based on 6-year lung cancer incidence, they identified a threshold above which NLST lung cancer mortality rates in the CT arm were consistently lower than in the CXR arm. They also evaluated the USPSTF and PLCOm2012 risk-based selection criteria in smokers (n=37,327) enrolled in the Prostate, Lung, Colorectal and Ovarian Cancer Screening (PLCO) Trial (32). The authors calculated the numbers of individuals selected for screening, sensitivity, specificity, and positive predictive values (PPVs) for identifying those subjects with lung cancer. At a PLCOm2012 measured risk of ~1.5% or more (the ~65th percentile of risk), the lung cancer mortality rates in the CT-screened arm are consistently below those in the CXR arm (33). The number needed to screen (NNS) to prevent one lung cancer death is significantly more favorable as the risk of lung cancer increases (Figure 1). When applied to PLCO intervention arm smokers, compared to the USPSTF criteria, the PLCOm2012 risk threshold of ~1.5% selects 8.8% fewer individuals for screening but identifies 12.4% more lung cancers. Ideally, in a shared decision-making context, use of a decision aid that allows patients to see their risk in absolute terms would permit consideration of the benefits of screening in a more informed way.
Caverly and colleagues considered how patient preferences might impact the benefits of screening by incorporating preference-sensitive adjustments to quality of life (disabilities) in a screening model. They found that the benefit of screening in terms of quality adjusted life years was sensitive to patient preferences at the extremes of risk (34). Patients with strongly unfavorable preferences regarding screening (i.e., higher screening-related disutility or stronger negative feelings) still experienced net benefit if lung cancer risks were high. Screening-related gains in quality-adjusted life years (QALY) peaked at a lung cancer risk near the ~70th percentile and began to decline at higher risk levels, mainly because of competing mortality risk in this group from cardiovascular and other pulmonary diseases. Although patient preferences also had a major impact on the net benefit of low-dose computed tomography (LDCT) screening, persons with lung cancer risk between 28th (1.8% 6-year risk) and 91st percentile (~9% 6-year risk) still receives net benefit even assuming attitudes that are highly unfavorable toward screening. This suggests that eliciting some measure of patient preferences in the context of lung cancer screening can be helpful in maximizing the benefits and reducing the harms of screening.
What are the risks of screening?
Surveys suggest that both patients, and providers are poorly informed about the risks of screening, and may overestimate some risks, while underestimating others (35). The chief risks of lung cancer screening include the very low risk of radiation, which cannot be measured directly and can only be estimated on a population level. The actual risk to any one individual from several years of low-dose CT screening is small (36,37). The most common risk of screening is that of a false positive which occurred in 36% of participants in the NLST over three rounds of screening (7), though with more nuanced reporting this problem too can be minimized (38-41). Ninety-six percent of lung nodules identified in the NLST were not cancer. While incidental lung nodules have been vexing problem for years, the added anxiety for smokers already worried about cancer, from the discovery of a screen detected lung nodule was a major concern of the designers of the NLST. Survey results from patients who are participants in the trial did not show an increase in anxiety, or decrease in quality of life, as a result of finding a pulmonary nodule (42). However, it should be remembered that these patients were being cared for in high volume centers likely by experts who had experience in managing “nodule anxiety”. Investigators from the NELSON trial also incorporated strategies to minimize scan related anxiety in their participants (Harry J. de Koning, Erasmus Medical Center, Public Health Rotterdam, IASLC World Congress, Plenary presentation, Toronto, CA, 2018). Overall, while false positives are quite common in screened population, the anxiety surrounding false positives is manageable. Over-diagnosis, or perhaps more accurately, overtreatment of clinically insignificant lung cancers is estimated to have occurred in 10–18% of all lung cancers detected in the NLST (14,43). The wide range of estimates for over-diagnosis can be explained in part by the time horizon considered as well as how one factors in competing mortality. Nevertheless, as in any cancer screening situation, there is a risk of detection (and treatment) of cancers which would otherwise have remained undetected, and asymptomatic, through the span of a patient’s life, had screening not been performed.
The risk of screening that concerns me the most is the risk of invasive procedures for benign disease. Results of the NLST showed that 1 in 40 participants (25 per 1,000) had an invasive biopsy for what proved to be benign disease (7). If this number goes up, or if the complication rate from biopsy is higher in routine practice than it was in this clinical trial, which clearly appears to be the case (22), then the net harm to the population could be significant. Efforts to maximize the benefits of screening, and minimize the harms, should therefore include strategies to reduce the number of invasive procedures done to investigate screen detected nodules, while still preserving the presumed benefits of “early detection”.
Who should not be screened?
An in-depth discussion of people for whom screening should not be offered, or for whom it should be frankly discouraged is almost certain to touch off some debate. However, when screening is viewed as a population-based intervention some discussion of the consequences of screening people outside the designated groups described above is possible. In this context, one can focus on the population-based outcomes anticipated as a result of widespread screening. First, based on the results of the NLST, one can conclude that the number of patients needed to be screened to prevent one lung cancer death significantly increases at the low end of risk. Post-hoc analyses show that when risk is broken down into quintiles, the absolute number of lung cancer death prevented by screening the lowest risk quintile was very low, with a NNS to prevent one death of >5,000 (33). However, the rate of false positives does not seem to vary significantly by quintiles of risk (33). Therefore, insofar as false positives serve as a surrogate for harm (e.g., invasive procedures on people with benign disease), the ratio of potential harm to benefit in people who are low risk for cancer should create caution in offering screening. The number false positive results per death avoided by screening decreased from 108 overall to 78 in the three highest-risk quintiles, but it was over 1,600 in the lowest risk quintile (33).
On the other end of the spectrum, people with significant comorbid disease that limits their life expectancy have much less to gain from “early diagnosis” of lung cancer. Screening for cancer is, in effect, an effort to increase the probability of death from another cause. Patients with significant comorbid disease, such as advanced [e.g., Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage III or IV] COPD, or severe cardiovascular disease expected to limit their lifespan, are much more likely to die of non-cancer related cause, perhaps even in the presence of cancer. Additionally, the risk to such individuals of invasive procedures required to evaluate abnormal screen-related findings is much greater than those who are in otherwise good health. Examples of individuals being offered screening when they already suffer from significant comorbid disease (44) suggest that this is not an isolated problem, and that additional education of healthcare professionals on who should and should not be screened would be useful. Clinical models that allow rational consideration of competing mortality risks in the context of cancer screening would be helpful. The promise of electronic health record prompts to promote screening for appropriate individuals or discourage it for individuals in whom it would be inappropriate has not yet been realized. One would hope that as computer-based health records improve, this sort of decision support would be an important outcome.
How should screening be done?
Lung cancer screening CT scans should be performed using low radiation dose, with interpretation by qualified thoracic radiology specialists. At present, screening is recommended annually for those eligible to be screened, as long as they remain in good health. Some investigators have made efforts to identify people who might still benefit from less frequent screens. For example, Berg and colleagues reported, in abstract form, the conditional probability of finding lung cancer after a negative incidence screen, and showed that the probability of cancer is reduced in those with a negative baseline screen (45). It is possible that in the future, screening recommendations may shift to less frequent scans for those with negative baseline screens. This strategy would have to be very carefully validated, to assure that screening benefits are not lost with less frequent scans.
Lung cancer screening with low-dose CT is intended to find early stage, but potentially fatal lung cancer, and do so at a point when surgical resection is curable. For now, this means detection of lung nodules, the vast majority of which are benign. Radiographic tools to distinguish benign from malignant nodules are extremely inaccurate, such that nodule management guidelines currently focus on determining the probability of cancer based on features of the nodule as well as the patient (46,47). This permits clinicians to follow probability-based algorithms to determine which nodules should be followed with interval CT scans, which should be biopsied, and which patients should be referred for surgical resection. In-depth discussion of the management of lung nodules in the context of screening is beyond the scope of this review, but important principles should be considered; (I) Since the vast majority of nodules are benign, emphasis should be placed on conservative management until there is evidence of a high probability of malignancy, and (II) Invasive procedures for screen detected nodules should be done in centers with multidisciplinary teams of clinicians with significantly high volume of experience in these procedures, particularly if surgery is considered. It is important to recall that the surgical morality for lung cancer resection in subjects enrolled in the NLST was 1%. This (or better) should be the goal for any center attempting to conduct a lung cancer screening program, and surgical mortality for screen detected lung nodules should be one of several important quality metrics for such programs. The American College of Chest Physicians and the American Thoracic Society have developed a joint policy statement describing the necessary components of a high-quality lung cancer screening program. Prominent among these recommendations is the need to track surgical outcomes (Table 2) (10).

Full table
The NLST trial did not specify a follow-up protocol for abnormal findings. However, currently in the U.S., the CMS currently requires that programs report the results of screening CT scans using a structured format that includes (among many other things) recommended intervals of follow-up for abnormal scans. The most commonly used structure format relies on the Lung-RADS criteria, which has been shown to significantly reduce the “false positive” rate (38), by increasing the threshold for a positive screen to a nodule ≥6 mm. Doing so substantially reduces the false positive rate while still capturing nearly 100% of lung cancers. This change in criteria are only valid the context of a screening program in which nodules smaller than this would routinely be followed with an annual scan anyway. For individuals with incidental nodules discovered outside of the screening context, the proper follow up guidelines would be the updated Fleischner criteria (48).
What have we learned from the release of data from NELSON trial mortality data? The mortality results for the largest European lung cancer screening trial to date were presented at the International Association for the Study of Lung Cancer (IASLC) in 2018 (15). This study enrolled over 15,000 subjects with tobacco-use criteria that were somewhat lower than the eligibility criteria for the NLST (>10 cigarettes/day for 30 years, or >15/day for 25 years). It also enrolled a lower percentage of women than were enrolled in NLST. In spite of these differences which would tend to identify a lower-risk population, the mortality reduction of at least 26% seen in NELSON was greater than what was observed by NLST investigators. What may account for these differences? It is possible that ethnic and genetic differences account for some of the disparity. However, it is also important to acknowledge the substantially different strategy employed in the NELSON trial for identifying normal versus abnormal CT scans and the management of indeterminate nodules. Rather than relying on simple two-dimensional measurements of nodule size, investigators in the NELSON trial employed volumetric measurements of pulmonary nodules, and created more detailed categories of CT findings; negative scans (without a nodule, or with nodules less than 50 mm3), positive scans (solid nodules >500 mm3), and indeterminate scans (nodules 50–500 mm3 in size). Unlike the binary reporting of scan results in the NLST, the NELSON trial reporting strategy included positive, and negative scan results, as well as allowing for indeterminate results which required a short-term follow-up. Subjects with nodules in the indeterminate category were prompted for a repeat scan 3–4 months later to assess growth using volumetric (not diameter) measurements. If there was no significant growth on the repeat scan, the test result was re-classified as negative and participants were scheduled for an annual repeat CT scan 8–9 months later. If there was significant growth, the test result was then categorized as positive, and a histologic diagnosis had to be obtained. It is possible that this difference in nodule management proscribed by the NELSON trial protocol has something to do with the more robust mortality benefit observed in the NELSON trial (compared to NLST). Investigators should be focused on determining how this contributed to the different outcomes of these two very important trials. Nodules >500 mm3 in the NELSON trial were considered positive, and required referral for work-up and diagnosis. This nodule management strategy reduced false positives while maintaining a low rate (2.3%) of referral to specialists for suspicious nodules. Investigators in both Europe and the U.S. have proposed that nodule volume measurements can aid in evaluating pulmonary nodules, and improve the predictive power of existing models (41,49). In the NELSON trial there was a high rate of benign disease detected among subjects who had surgery for a screen-detected nodule (50), and though the surgical mortality in NELSON was very low, this finding should prompt caution in relying solely upon volumetric measurements as an indication for surgical resection. Benign nodules can grow as well.
What other factors will impact the risk:benefit ratio of lung cancer screening?
Beyond the identification and management of pulmonary nodules, there are other factors which almost certainly will impact the overall population benefit of a lung cancer screening program. Quality metrics which are outlined in the American College of Chest Physicians/American Thoracic Society (ACCP/ATS) position statement include factors such as training of radiologists in the interpretation in reporting of scans. CT scans done for lung cancer screening should be interpreted by board certified thoracic radiologists. Nodule evaluations should be carried out by pulmonologists with extensive experience in the management of pulmonary nodules. While it is important to know how to do a biopsy or surgery, it may be more important to know when not to. Nodules with a low probability of cancer should not be biopsied in general. A biopsy can only prove the presence of cancer, not disprove it. It is also critically important that surgical resection of screen detected lung cancers be done by highly experienced thoracic surgeons who can demonstrate low risk-adjusted mortality for their patients. If surgical mortality were to go from 1%, as in the NLST, to 2%, which is still better than the national average, it could negate a substantial portion the mortality benefit from lung cancer screening. On the other hand, as rates of minimally invasive lung cancer surgery increase, the risk of surgery should continue to go down, particularly in the hands of experienced thoracic surgical oncologists. Most lung cancer screening trials were conducted during a time when rates of minimally invasive surgery were gradually increasing, and the current environment for thoracic surgical treatment of lung cancer is probably safer, as a reflection of this overall trend. Only ~30% of lung cancer resections in the NLST were done with a minimally invasive approach (51). As minimally invasive surgery increasingly replaces open thoracotomy as the standard of care, one can expect improved outcomes from resection and screen detected lung cancer as a result of this trend. Increased capacity of trained thoracic surgeons may be needed to manage the burden of lung cancer screening (52).
Additional factors can be expected to improve the ratio of benefits to harm in lung cancer screening. One example of this would be the active, and ongoing search for a diagnostic biomarker for both incidental, and screen detected lung nodules. An exhaustive review of these potential biomarkers is beyond the scope of this manuscript, but it is easy to envision that a biomarker with a very high negative predictive value can have substantial benefit for those with screen detected nodules, in terms of avoiding unnecessary invasive procedures. There will always be a trade-off between such a biomarker, and the potential delay in the diagnosis of an otherwise malignant lesion, in the case of a false negative result from such a test. But the net benefit from a population level will be measured by reduced harm, while preserving the benefits of the screening process (53). Interested readers are encouraged to review these references discussing the current search for diagnostic biomarkers (53,54).
The importance of tobacco cessation
The importance of incorporating tobacco cessation into mass lung cancer screening programs cannot possibly be overstated. Tobacco cessation alone has a greater mortality benefit than screening does, but when combined with tobacco cessation, the cost-effectiveness (55), and clinical impact of lung cancer screening is amplified. It may also be true that lung cancer screening represents the critical teachable moment required to overcome the ambivalence some smokers have to a cessation attempt (56-58). Numerous clinical guidelines highlight the importance of, and strategies for, incorporating effective tobacco cessation treatment into lung cancer screening programs (10,11,13,14). While ongoing clinical trials attempt to address this problem (59), there is currently little data, and not much consensus on the optimal means of delivering tobacco cessation counseling, and/or pharmacotherapy in the context of a lung cancer screening program. There are, nevertheless, numerous excellent guidelines for tobacco cessation counseling and pharmacotherapy (60-63).
Summary
The widespread implementation of lung cancer screening will take some time, but it has the potential to significantly reduce the mortality burden of the leading cancer related killer in the world. The tobacco industry has brought an enormous amount of suffering and death upon its victims, and lung cancer screening is one way to potentially reduce this suffering. There are key components of a lung cancer screening program which should be observed when implementing lung cancer screening on a population basis. These include careful review, and structured reporting of CT results. Responsible follow-up of abnormal findings by experts in the management of lung nodules, and, whenever possible, referral to centers with significant experience in lung cancer surgery for those requiring treatment of screen detected lung cancers. Tobacco cessation practices should be implemented in all settings, but particularly in the context of a lung cancer screening program, where it may be expected to have a greater impact.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Fontana RS, Sanderson DR, Woolner LB, et al. Lung cancer screening: the Mayo program. J Occup Med 1986;28:746-50. [Crossref] [PubMed]
- Melamed MR, Flehinger BJ, Zaman MB, et al. Screening for Early Lung Cancer: Results of the Memorial Sloan-Kettering Study in New York. Chest 1984;86:44-53. [Crossref] [PubMed]
- Frost JK, Ball WC Jr, Levin ML, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis 1984;130:549-54. [PubMed]
- Levin ML, Tockman MS, Frost JK, et al. Lung Cancer Mortality in Males Screened by Chest X-ray and Cytologic Sputum Examination: A Preliminary Report. In: Band PR. editor. Early Detection and Localization of Lung Tumors in High Risk Groups. Berlin, Heidelberg: Springer Berlin Heidelberg, 1982:138-46.
- Kubik A, Parkin DM, Khlat M, et al. Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer 1990;45:26-33. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Kinsinger LS, Anderson C, Kim J, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA Intern Med 2017;177:399-406. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest 2018;153:954-85. [Crossref] [PubMed]
- Tanoue LT, Tanner NT, Gould MK, et al. Lung cancer screening. Am J Respir Crit Care Med 2015;191:19-33. [Crossref] [PubMed]
- Wiener RS, Gould MK, Arenberg DA, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med 2015;192:881-91. [Crossref] [PubMed]
- Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- De Koning H, Van Der Aalst C, Ten Haaf K, et al. PL02.05 Effects of Volume CT Lung Cancer Screening: Mortality Results of the NELSON Randomised-Controlled Population Based Trial. J Thorac Oncol 2018;13:S185. [Crossref]
- Wegwarth O, Schwartz LM, Woloshin S, et al. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med 2012;156:340-9. [Crossref] [PubMed]
- Lewis JA, Petty WJ, Tooze JA, et al. Low-Dose CT Lung Cancer Screening Practices and Attitudes among Primary Care Providers at an Academic Medical Center. Cancer Epidemiol Biomarkers Prev 2015;24:664-70. [Crossref] [PubMed]
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012;10:240-65. [Crossref] [PubMed]
- Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer 2001;92:153-9. [Crossref] [PubMed]
- Swensen SJ, Jett JR, Hartman TE, et al. CT Screening for Lung Cancer: Five-year Prospective Experience. Radiology 2005;235:259-65. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771-9. [Crossref] [PubMed]
- Huo J, Xu Y, Sheu T, et al. Complication Rates and Downstream Medical Costs Associated With Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting. JAMA Intern Med 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Infante M, Cavuto S, Lutman FR, et al. Long-Term Follow-up Results of the DANTE Trial, a Randomized Study of Lung Cancer Screening with Spiral Computed Tomography. Am J Respir Crit Care Med 2015;191:1166-75. [Crossref] [PubMed]
- Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with Focus on High-Risk Profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Physicians. AAoF. Clinical preventive service recommendation: lung cancer. Leawood, KS: American Academy of Family Physicians; 2013. A. 2013. Available online: http://www.aafp.org/patient-care/clinical-recommendations/all/lung-cancer.html. Accessed Jan 19 2019.
- Gerber DE. Should family physicians routinely screen for lung cancer in high-risk populations? Yes: CT-based screening is complex but worthwhile. Am Fam Physician 2014;90:73B-4B. [PubMed]
- Seehusen DA. Should family physicians routinely screen for lung cancer in high-risk populations? No: The USPSTF's recommendation for lung cancer screening is overreaching. Am Fam Physician 2014;90:73D-4D. [PubMed]
- Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003;95:470-8. [Crossref] [PubMed]
- Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270-6. [Crossref] [PubMed]
- Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst 2011;103:1058-68. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764. [Crossref] [PubMed]
- Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. [Crossref] [PubMed]
- Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245-54. [Crossref] [PubMed]
- Caverly TJ, Cao P, Hayward RA. Ann Intern Med 2018;169:1-9. [Crossref] [PubMed]
- Hoffman RM, Sussman AL, Getrich CM, et al. Attitudes and Beliefs of Primary Care Providers in New Mexico About Lung Cancer Screening Using Low-Dose Computed Tomography. Prev Chronic Dis 2015;12:E108. [Crossref] [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. Erratum in: JAMA 2013;309:2212. JAMA 2012;308:1324. [Crossref] [PubMed]
- Albert JM. Radiation risk from CT: implications for cancer screening. AJR Am J Roentgenol 2013;201:W81-7. [Crossref] [PubMed]
- Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162:485-91. [Crossref] [PubMed]
- Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Vliegenthart R, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013;42:1659-67. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Gareen IF, Duan F, Greco EM, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014;120:3401-9. [Crossref] [PubMed]
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. Erratum in: JAMA Intern Med 2014;174:828. [Crossref] [PubMed]
- Schneider D, Arenberg D. Competing mortality in cancer screening: a teachable moment. JAMA Intern Med 2015;175:896-7. [Crossref] [PubMed]
- Robbins HA, Berg C, Cheung LC, et al. Effect of Screening CT Findings on Lung Cancer Risk Prediction Within the National Lung Screening Trial. Am J Respir Crit Care Med 2017;195:A7056.
- Baldwin DR, Callister ME. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest 2014;145:464-72. [Crossref] [PubMed]
- Van't Westeinde SC, Horeweg N, De Leyn P, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg 2012;42:420-9. [Crossref] [PubMed]
- Kamel MK, Lee B, Harrison S, et al. Do the surgical results in the National Lung Screening Trial reflect modern thoracic surgical practice? J Thorac Cardiovasc Surg 2018. [Epub ahead of print].
- Blom EF, Ten Haaf K, Arenberg DA, et al. Treatment capacity required for full-scale implementation of lung cancer screening in the United States. Cancer 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Mazzone PJ, Sears CR, Arenberg DA, et al. Evaluating Molecular Biomarkers for the Early Detection of Lung Cancer: When Is a Biomarker Ready for Clinical Use? An Official American Thoracic Society Policy Statement. Am J Respir Crit Care Med 2017;196:e15-29. [Crossref] [PubMed]
- Seijo LM, Peled N, Ajona D, et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol 2019;14:343-57. [Crossref] [PubMed]
- Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793-802. [Crossref] [PubMed]
- van der Aalst CM, van den Bergh KA, Willemsen MC, et al. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600-5. [Crossref] [PubMed]
- Park ER, Gareen IF, Jain A, et al. Examining whether lung screening changes risk perceptions: National Lung Screening Trial participants at 1-year follow-up. Cancer 2013;119:1306-13. [Crossref] [PubMed]
- Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084. [Crossref] [PubMed]
- Joseph AM, Rothman AJ, Almirall D, et al. Lung Cancer Screening and Smoking Cessation Clinical Trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med 2018;197:172-82. [Crossref] [PubMed]
- Anderson JE, Jorenby DE, Scott WJ, et al. Treating tobacco use and dependence: an evidence-based clinical practice guideline for tobacco cessation. Chest 2002;121:932-41. [Crossref] [PubMed]
- Shields PG, Herbst RS, Arenberg D, et al. Smoking Cessation, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1430-68. [Crossref] [PubMed]
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017;3:CD001292. [PubMed]
- Fiore M, Jaen CR, Baker T, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services, 2008.


